
J. Cent. South Univ. (2020) 27: 155-163
DOI: https://doi.org/10.1007/s11771-020-4285-2
Variability of leaf functional traits of invasive tree Rhus typhina L. in North China
WEI Mei(韦梅)1, WANG Shu(王舒)1, WU Bing-de(伍丙德)1, JIANG Kun(姜坤)1,ZHOU Jia-wei(周嘉伟)1, 2, WANG Cong-yan(王从彦)1, 3
1. Institute of Environment and Ecology, Academy of Environmental Health and Ecological Security & School of the Environment and Safety Engineering, Jiangsu University, Zhenjiang 212013, China;
2. School of the Environment, Nanjing University, Nanjing 210023, China;
3. State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
Functional traits, specifically leaf functional traits, are core-topics to explore importance to the invasion success of invasive plant species. This study aims to address the differences in leaf functional traits and their corresponding variability of the invasive tree staghorn sumac Rhus typhina L. with different invasion success, including lower and higher invasion success, in two climatic regions in North China, including a warm temperate region and a cold temperate region. No significant differences were found for leaf functional traits of staghorn sumac across different invasion success. However, the variability of leaf chlorophyll and nitrogen concentrations of staghorn sumac under higher invasion success were approximately 66.023% and 68.615% higher than those under lower invasion success, respectively. The leaf chlorophyll and nitrogen concentrations of staghorn sumac in the warm temperate region were approximately 18.432% and 16.337% higher than those in cold temperate region, respectively. The variability of specific leaf area of staghorn sumac in warm temperate region was approximately 59.802% higher than that in cold temperate region. Accordingly, leaf chlorophyll and N concentrations as well as specific leaf area of staghorn sumac and their corresponding variability may play an essential role in shaping ecological success of studied invader along a climatic gradient.
Key words:
Cite this article as:
WEI Mei, WANG Shu, WU Bing-de, JIANG Kun, ZHOU Jia-wei, WANG Cong-yan. Variability of leaf functional traits of invasive tree Rhus typhina L. in North China [J]. Journal of Central South University, 2020, 27(1): 155-163.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4285-21 Introduction
Invasive plant species (invaders hereafter) have an enormous impact on the structure and function of the local ecosystems. Thus, the invasion mechanisms of successful invaders have stimulated considerable research interest [1-4]. Functional traits, specifically leaf functional traits play a key role in the resource acquisition and utilization and the corresponding competitiveness of plant species, including the invasiveness of the invaders [5-9]. Specific leaf area (SLA), an essential functional trait, can be a major factor in the trade-offs between resource acquisition and conservation [10-12] as well as the trade-offs between competitive and stress-tolerant [2, 3, 13]. Leaves with higher SLA are typically associated with higher resource use efficiency and acquisition as well as lower structural investment; in contrast, leaves with lower SLA often correspond to mass investment in leaf biomass and relatively lower growth rates [10-12]. In addition, leaf size, leaf shape index, leaf chlorophyll and nitrogen (N) concentrations, leaf thickness, and single-leaf fresh and dry weights are also fundamental indices to be evaluated since they can be effectively used as a proxy for the resource-use strategy of plant species [5, 14-16].
The successful invasion process of invaders in a new ecosystem is generally gradual after they are transported from their original locations [15, 17-20]. Thus, there are progressive changes in the relative abundances of invaders, and these invaders exhibit a gradient of invasion success in the colonized ecosystem [15, 18, 20]. Moreover, the introduced range of some invaders may cover several climatic regions [20-22]. Thus, it is important to understand the variations in the key functional leaf traits along a climatic gradient that may be related to resource use strategies of invaders to better understand the ecological strategies that are at the base of its successful invasion process.
In this study, cross-site comparisons were performed to determine differences in leaf functional traits and their corresponding variability of invaders in plant communities with different invasion success in two climatic regions in North China. In this study, the notorious invasive tree staghorn sumac Rhus typhina L. was selected as the targeted invader.
We examine the following hypotheses. First, all leaf functional traits of staghorn sumac may be higher in warm temperate region than in cold temperate region because of the lower temperature, precipitation, and light intensity in the cold temperate region can inhibit the growth of plant species [23-25]. Second, the SLA of staghorn sumac increases with increasing invasion success. Invaders may allocate a greater fraction of biomass to leaf growth to attain a higher growth rate with a higher invasion success under competitive conditions because intraspecific competition may progressively increase with increasing invasion success [15, 20, 26]. Third, the variability of leaf functional traits of the invader increases with the increase in invasion success. This is due to the fact that plant species show greater variability in response to shifts in environmental factors that contribute to the performance and fitness [2, 27-29]. Previous research revealed that the invasiveness of invaders was positively associated with the corresponding variability of functional traits [4, 27, 30].
2 Materials and methods
2.1 Focal species and study areas
Staghorn sumac is a deciduous tree in the family Anacardiaceae [21, 31]. The species is native to North America and was introduced into China in 1959 as a common forestry species [32, 33]. It has spread to most areas of North China currently [32-34]. This species is often used to restore the ecosystem in the degraded lands in most mountain areas of North China owning to its abundant growth, even in barren habitats [32, 34]. In addition, staghorn sumac is used as a showy ornamental plant species because of its fruit clusters, which look like flaming torches, in late summer and early autumn as well as having dazzling red leaves in mid-autumn [32, 35]. The species has some basic invasive characteristics such as rapid growth and high reproductive capacity [32, 34]. In particular, staghorn sumac is commonly distributed across multiple habitats from urban to montane [32] and has been recognized as a destructive invader in North China [32-34].
Plant samples of staghorn sumac were obtained from two sampling regions with different climates in North China. A warm temperate region, Jinan, included three sampling sites: Lake Daming (36.681°N 117.039°E), Thousand-Buddha Mountain (36.642°N 117.048°E), and Overpass of Mountain La (36.661°N 116.943°E). The annual mean temperature at the region is approximately 13.9 °C. The annual precipitation is approximately 623.0 mm. The annual sunshine time is approximately 2405.8 h. A cold temperate region, Jinzhou, included three sampling sites: Mont Orchid Riverlet (41.086°N 121.147°E), the vicinity of Nanshan Tunnel (41.065°N 121.140°E), and the vicinity of South Jinzhou Railway Station (41.031°N 121.125°E). The annual mean temperature at the region is approximately 9.1°C. The annual precipitation at this area is approximately 570 mm. The annual sunshine time is approximately 2715 h. The basic climate summaries of the two sampling regions are derived from their local climate records [36, 37]. Sampling sites were chosen in wasteland and there are no other trees except for staghorn sumac. The type of soils in the invaded habitats was yellow soil [38]. The results may be influenced by plot-specific effects, which were not included in the models.
2.2 Study design
Leaf samples of staghorn sumac were collected from each sampling site in early August, 2016. The invasion success of staghorn sumac was evaluated based on its overage (i.e., canopy coverage) in the invaded ecosystems and was divided into the following two categories: lower (<35%) and higher invasion success (>75%) [15, 20, 26]. In particular, the lower invasion success mimicked the colonization stage of the invasion process and the higher invasion success mimicked the landscape spread stage of the invasion process [18, 19]. Each sampling site consisted of a lower invasion success plot (three 2 m×2 m quadrats) and a higher invasion success plot (three 2 m×2 m quadrats). Five fully expanded, mature, and undamaged leaves in the sunny part of staghorn sumac were collected from each quadrat randomly selected adult individual plants to minimize the effects of sunlight on leaf functional traits. Functional traits were evaluated for each of the replicate leaf samples.
2.3 Determination of leaf functional traits and their corresponding variability of staghorn sumac
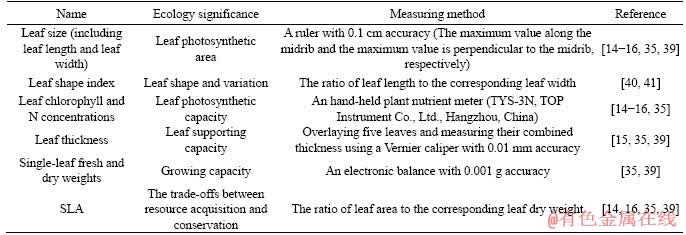
Nine parameters of leaf functional traits of staghorn sumac were determined. The name, ecological significance, measuring method, and the corresponding references for the evaluated parameters of leaf functional traits of staghorn sumac are shown in Table 1.
The coefficient of variation (CV) was calculated as CV=σ/μ to determine the variability of leaf functional traits of staghorn sumac, where μ represents the average value of one leaf functional trait of staghorn sumac in one particular quadrat and σ represents the standard deviation for the average value of the same leaf functional trait of staghorn sumac in the same particular quadrat [42, 43]. In particular, a higher value of CV represents a greater variability.
2.4 Statistical analysis
Data were evaluated to determine the deviations from normality and homogeneity of the variances before data analysis. Differences among leaf functional traits and their corresponding variability of staghorn sumac with differing invasion success and climatic regions were assessed using analysis of variance (ANOVA). Two-way ANOVA was used to evaluate the effects of invasion success and climatic region on leaf functional traits of staghorn sumac. Meanwhile, the partial eta squared (η2) values were also computed to evaluate the effect size of each factor for use in two-way ANOVA [44, 45]. A probability value of P≤0.05 was used to determine statistically significant differences. All statistical analyses were implemented in IBM SPSS Statistics (version 22.0; IBM Corp., Armonk, NY, USA).
Table 1 Name, ecological significance, measuring method, and corresponding references for assessed parameters of leaf functional traits of staghorn sumac
3 Results
No significant differences were found for all leaf functional traits of staghorn sumac across different invasion success (P>0.05; Figure 1 and Table 2). The leaf chlorophyll and N concentrations of staghorn sumac in the warm temperate region were approximately 18.432% and 16.337% higher than those in the cold temperate region, respectively (P<0.05; Figure 1 and Table 2). No significant differences were found in the remaining of present indices of leaf functional traits of staghorn sumac from the two climate regions (P>0.05; Figure 1 and Table 2).
The coefficient of variation of leaf chlorophyll and N concentrations of staghorn sumac under higher invasion success were approximately 66.023% and 68.615% higher than those under lower invasion success (P<0.05; Figure 2). The difference in the coefficient of variation for the remaining of present indices of leaf functional traits of staghorn sumac with different invasion success was not significant (P>0.05; Figure 2). The coefficient of variation of SLA of staghorn sumac in the warm temperate region was approximately 59.802% higher than that in the cold temperate region (P<0.05; Figure 2). There was no significant difference in the coefficient of variation of the remaining of present indices of leaf functional traits of staghorn sumac from the two climate regions (P>0.05; Figure 2).
4 Discussion
Previous studies revealed that higher temperatures and precipitation at southern regions may result in increases in SLA [10, 46, 47]. However, no significant differences were found in SLA of staghorn sumac from the two climate regions. Meanwhile, the relationship between SLA and climatic region was not consistent in previous studies, i.e., SLA may be higher in the colder temperate regions than in the warmer temperate regions [11, 47, 48]. These inconsistent results may arise from the different spatial scales used in those studies. In addition, the leaf chlorophyll and N concentrations of staghorn sumac in the warm temperate region were significantly greater than those in the cold temperate region. This finding was consistent with the study’s first hypothesis. This finding may be the result of the lower temperature, precipitation, and light intensity in the cold temperate region that can inhibit the growth of staghorn sumac [23-25]. Further, the difference in leaf chlorophyll concentration of staghorn sumac in the two climate regions could produce a profound effect on the capture and use of sunlight which is an essential ecological factor that affects plant establishment, growth, and survival [7, 8, 49]. The difference in leaf functional traits of staghorn sumac the two climate regions may represent the differentiation of adaptive strategies in response to latitudinal shifts in temperature, precipitation, and sunlight availability.
Plant species can exhibit variability, to a certain extent, in response to shifts in environmental factors [4, 27-29]. In particular, the increased variability of plant species for any functional trait can make a broader upgrade of their performance and fitness and thus may play an essential role in their successful ecological strategy [2, 11, 27]. More importantly, the invasiveness of invaders has been positively related to the variability of functional traits [4, 27, 30]. Leaf chlorophyll and N concentrations of staghorn sumac had variability within the range of environmental factors in this study. This finding implied that the two leaf functional traits that display a particular range of variability may play an important role in successful ecological strategies which enable staghorn sumac to locally adapt to the various environments across different invasion success and climatic regions through a widened habitat niche. Predictably, the variability of the two leaf functional traits of staghorn sumac increased with a higher invasion success. The results of this study also revealed that the variability of SLA of staghorn sumac increased with a heavy invasion success but did not differ significantly from a light invasion success. However, the variability of leaf chlorophyll and N concentrations of staghorn sumac significantly increased with a heavy invasion success. This result was consistent with the study’s third hypothesis. The higher range of variability of leaf chlorophyll and N concentrations of staghorn sumac under higher invasion success may favor increased resource (especially sunlight) capture and maximized efficiency during growth, enabling the species to establish a colony in a competitive environment [14, 49, 50].

Figure 1 Leaf functional traits of staghorn sumac with different invasion success in two climatic regions (Bars (mean±SE, n=18) with different lowercase letters indicate a significant difference (P<0.05) within the same group (Climatic region/Invasion success). “ns” means no significant difference (P>0.05). The P and F values per subfigure represent the statistical significance level among all treatment groups per parameter)
Consistent with greater abiotic filtering (especially less intense sunlight and lower temperatures) at higher latitudes [51], the results of this study showed that the coefficient of variation of SLA of staghorn sumac was significantly higher in the warm temperate region than in the cold temperate region. The higher range of variability of SLA of staghorn sumac in the warm temperate region may be because staghorn sumac in the warm temperate region is better able to adapt to the shifting environment by adjusting the resource investment per unit area and per lamina. Previous results also presented that certain invaders should tolerate biotic resistance better than others because they possess functional traits that allow them to cope with competition more efficiently [2]. The reason for this phenomenon may also be due to the fact that the warm temperate region in North China in which staghorn sumac vigorously occurs has the similar climatic conditions with its natural habitat in the original distribution area.
Table 2 Two-way ANOVA on effects of invasion success and climatic region on leaf functional traits of staghorn sumac
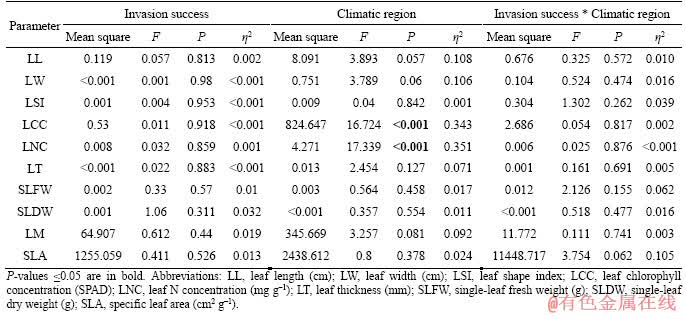
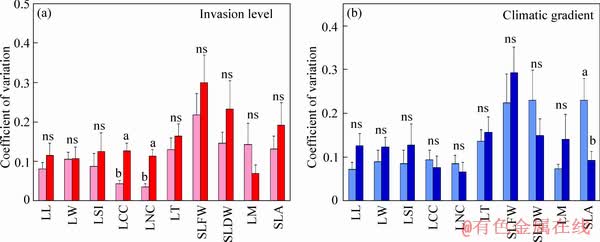
Figure 2 Coefficient of variation of leaf functional traits of staghorn sumac with different invasion success in two climatic regions (lower invasion success, light red bars; higher invasion success, heavy red bars; warm temperate region, light blue bars; cold temperate region, heavy blue bars). Abbreviations have the same meanings as described in Table 2. Bars (mean±SE, n=18) with different lowercase letters indicate a significant difference (P<0.05). “ns” means no significant difference (P>0.05)
The results of this study may contribute to the understand of the mechanism driving the success of staghorn sumac invasion, especially across many climatic zones. However, only two climate regions were studied to determine leaf functional traits and their corresponding variability of staghorn sumac in this study. This is the deficiency of the experimental design in this study. Thus, more climate regions and/or gradients will be adopted in our future research to obtain more contents about the differences in leaf functional traits and their corresponding variability of staghorn sumac.
5 Conclusions
Overall, the leaf chlorophyll and N concentrations of staghorn sumac in the warm temperate region were significantly higher than those in cold temperate region. The variability of specific leaf area of staghorn sumac in warm temperate region was significantly higher than that in cold temperate region. Meanwhile, the variability of leaf chlorophyll and nitrogen concentrations of staghorn sumac under higher invasion success were significantly higher than those under lower invasion success although no significant differences were found for leaf functional traits of staghorn sumac across different invasion success. Thus, leaf chlorophyll and N concentrations as well as SLA of staghorn sumac and their corresponding variability may play an essential role in its invasion success along a climatic gradient.
References
[1] POWELL K I, CHASE J M, KNIGHT T M. Invasive plants have scale-dependent effects on diversity by altering species-area relationships [J]. Science, 2013, 339(6117): 316-318. DOI: 10.1126/science.1226817.
[2] CONTI L, BLOCK S, PAREPA M, MUNKEMULLER T, THUILLER W, ACOSTA A T R, VAN KLEUNEN M, DULLINGER S, ESSL F, DULLINGER I, MOSER D, KLONNER G, BOSSDORF O, CARBONI M. Functional trait differences and trait plasticity mediate biotic resistance to potential plant invaders [J]. Journal of Ecology, 2018, 106(4): 1607-1620. DOI: 10.1111/1365-2745.12928.
[3] TECCO P A, URCELAY C, DIAZ S, CABIDO M, PEREZ-HARGUINDEGUY N. Contrasting functional trait syndromes underlay woody alien success in the same ecosystem [J]. Austral Ecology, 2013, 38(4): 443-451. DOI: 10.1111/j.1442-9993.2012.02428.x.
[4] DYDERSKI M K, JAGODZINSKI A M. Functional traits of acquisitive invasive woody species differ from conservative invasive and native species [J]. NeoBiota, 2019, 41: 91-113. DOI: 10.3897/neobiota.41.31908.
[5] WRIGHT I J, ACKERLY D D, BONGERS F, HARMS K E, IBARRA-MANRIQUEZ G, MARTINEZ-RAMOS M, MAZER S J, MULLER-LANDAU H C, PAZ H, PITMAN N C A, POORTER L, SILMAN M R, VRIESENDORP C F, WEBB C O, WESTOBY M, WRIGHT S J. Relationships among ecologically important dimensions of plant trait variation in seven neotropical forests [J]. Annals of Botany 2007, 99(5): 1003-1015. DOI: 10.1093/aob/mcl066.
[6] SUDING K N, LAVOREL S, CHAPIN F S, CORNELISSEN J H C, DIAZ S, GARNIER E, GOLDBERG D, HOOPER D U, JACKSON S T, NAVAS M L. Scaling environmental change through the community- level: A trait-based response-and-effect framework for plants [J]. Global Change Biology 2008, 14(5): 1125-1140. DOI: 10.1111/j.1365-2486.2008.01557.x.
[7] LIU F D, YANG W J, WANG Z S, XU Z, LIU H, ZHANG M, LIU Y H, AN S Q, SUN S C. Plant size effects on the relationships among specific leaf area, leaf nutrient content, and photosynthetic capacity in tropical woody species [J]. Acta Oecologica-International Journal of Ecology, 2010, 36(2): 149-159. DOI: 10.1016/j.actao.2009.11.004.
[8] MENG F Q, CAO R, YANG D M, NIKLAS K J, SUN S C. Trade-offs between light interception and leaf water shedding: A comparison of shade- and sun-adapted species in a subtropical rainforest [J]. Oecologia, 2014, 174(1): 13-22. DOI: 10.1007/s00442-013-2746-0.
[9] CLELAND E E. Trait divergence and the ecosystem impacts of invading species [J]. New Phytologist, 2011, 189(3): 649-652. DOI: 10.1111/j.1469-8137.2010.03607.x.
[10] WRIGHT I J, REICH P B, WESTOBY M, ACKERLY D D, BARUCH Z, BONGERS F, CAVENDER-BARES J, CHAPIN F S, CORNELISSEN J H C, DIEMER M, FLEXAS J, GARNIER E, GROOM P K, GULIAS J, HIKOSAKA K, LAMONT B B, LEE T, LEE W, LUSK C, MIDGLEY J J, NAVAS M L, NIINEMETS U, OLEKSYN J, OSADA N, POORTER H, POOT P, PRIOR L, PYANKOV V I, ROUMET C, THOMAS S C, TJOELKER M G, VENEKLAAS E, VILLAR R. The worldwide leaf economics spectrum [J]. Nature, 2004, 428(6985): 821-827. DOI: 10.1038/nature02403.
[11] POORTER H, NIINEMETS U, POORTER L, WRIGHT I J, VILLAR R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis [J]. New Phytologist, 2009, 182(3): 565-588. DOI: 10.1111/j.1469-8137.2009. 02830.x.
[12] MAJEKOVA M, DE BELLO F, DOLEZ J, LEPS J. Plant functional traits as determinants of population stability [J]. Ecology 2014, 95(9): 2369-2374. DOI: 10.1890/13-1880.1.
[13] PIERCE S, BRUSA G, VAGGE I, CERABOLINI B E L. Allocating CSR plant functional types: The use of leaf economics and size traits to classify woody and herbaceous vascular plants [J]. Functional Ecology, 2013, 27(4): 1002-1010.
[14] WANG C Y, ZHOU J W, LIU J, JIANG K. Differences in functional traits between invasive and native Amaranthus species under different forms of N deposition [J]. Science of Nature, 2017, 104(7, 8): 59. DOI: 10.1007/s00114-017- 1482-4.
[15] WANG C Y, JIANG K, LIU J, ZHOU J W, WU B D. Moderate and heavy Solidago canadensis L. invasion are associated with decreased taxonomic diversity but increased functional diversity of plant communities in East China [J]. Ecological Engineering, 2018, 112(3): 55-64. DOI: 10.1016/j.ecoleng.2017.12.025.
[16] WANG C Y, WU B D, JIANG K, ZHOU J W. Differences in functional traits between invasive and native Amaranthus species under simulated acid deposition with a gradient of pH levels [J]. Acta Oecologica, 2018, 89: 32-37. DOI: 10.1016/j.actao.2018.04.006.
[17] WANG C Y, JIANG K, ZHOU J W, WU B D. Solidago canadensis invasion affects soil N-fixing bacterial communities in heterogeneous landscapes in urban ecosystems in East China [J]. Science of the Total Environment, 2018, 631-632: 702-713. DOI: 10.1016/ j.scitotenv.2018.03.061.
[18] THEOHARIDES K A, DUKES J S. Plant invasion across space and time, factors affecting nonindigenous species success during four stages of invasion [J]. New Phytologist 176(2): 256-273. DOI: 10.1111/j.1469-8137.2007.02207.x.
[19] RAI P K. Paradigm of plant invasion: multifaceted review on sustainable management [J]. Environmental Monitoring and Assessment, 2015, 187(12): 759. DOI: 10.1007/s10661- 015-4934-3.
[20] WANG C Y, ZHOU J W, LIU J, XIAO H G, WANG L. Functional traits and reproductive allocation strategy of Conyza canadensis as they vary by invasion degree along a latitude gradient [J]. Polish Journal of Environmental Studies 2017, 26(3): 1289-1297. DOI: 10.15244/pjoes/66175.
[21] YAN X L, LIU Q R, SHOU H Y, ZENG X F, ZHANG Y, CHEN L, LIU Y, MA HY, QI S Y, MA J S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants [J]. Biodiversity Science, 2014, 5: 667-676. DOI: 10.3724/SP.J.1003.2014. 14069. (in Chinese)
[22] WANG C Y, JIANG K, ZHOU J W, LIU J. Allelopathic suppression by Conyza canadensis depends on the interaction between latitude and the degree of the plant’s invasion [J]. Acta Botanica Brasilica, 2017, 31(2): 212-219. DOI: 0102-33062017abb0045.
[23] REICH P B, OLEKSYN J. Global patterns of plant leaf N and P in relation to temperature and latitude [J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(30): 11001-11006. DOI: 10.1073/pnas. 0403588101.
[24] SUN X Y, LU Z H, LI P H, JIANG Q S, LANG Z. Ecological adaptation of Eupatorium adenophorum populations to light intensity [J]. Journal of Forestry Research, 2006, 17(2): 116-120. DOI: 10.1007/s11676-006- 0027-z.
[25] MCCULLEY R L, BURKE I C, LAUENROTH W K. Conservation of nitrogen increases with precipitation across a major grassland gradient in the Central Great Plainsof North America [J]. Oecologia, 2009, 159(3): 571-581. DOI: 10.1007/s00442-008-1229-1.
[26] WANG C Y, ZHOU J W, LIU J, WANG L, XIAO H G. Reproductive allocation strategy of two herbaceous invasive plants across different cover classes [J]. Polish Journal of Environmental Studies, 2017, 26(1): 355-364. DOI: 10.15244/pjoes/64240.
[27] VAN KLEUNEN M, WEBER E, FISCHER M. A meta-analysis of trait differences between invasive and non-invasive plant species [J]. Ecology Letters, 2010, 13(2): 235-245. DOI: 10.1111/j.1461-0248.2009.01418.x.
[28] CHEN L Y, TIU C J, PENG S L, SIEMANN E. Conspecific plasticity and invasion: Invasive populations of Chinese tallow (Triadica sebifera) have performance advantage over native populations only in low soil salinity [J]. PLoS One, 2013, 8: e74961. DOI: 10.1371/journal.pone.0074961.
[29] GRIFFITH A B, ANDONIAN K, WEISS C P, LOIK M E. Variation in phenotypic plasticity for native and invasive populations of Bromus tectorum [J]. Biological Invasions, 2014, 16(12): 2627-2638. DOI: 10.1007/s10530-014- 0692-3.
[30] HULME P. Phenotypic plasticity and plant invasions: Is it all Jack? [J]. Functional Ecology, 2008, 22(1): 3-7. DOI: 10.1111/j.1365-2435.2007.01369.x.
[31] WANG C Y, LIU J, XIAO H G, ZHOU J W, DU D L. Floristic characteristics of alien invasive seed plant species in China [J]. Anais da Academia Brasileira de Ciências, 2016, 88(3): 1791-1797. DOI: 10.1590/0001-3765201620150687.
[32] WANG G, JIANG G, YU S, LI Y, LIU H. Invasion possibility and potential effects of Rhus typhina on Beijing Municipality [J]. Journal of Integrative Plant Biology, 2008, 50(5): 522-530. DOI: 10.1111/j.1744-7909.2008.00660.x.
[33] YUAN Y F, GUO W H, DING W J, DU N, LUO Y J, LIU J, XU F, WANG R Q. Competitive interaction between the exotic plant Rhus typhina L. and the native tree Quercus acutissima Carr. in Northern China under different soil N:P ratios [J]. Plant and Soil, 2013, 372(1, 2): 389-400. DOI: 10.1007/s11104-013-1748-3.
[34] ZHANG Z J, JIANG C D, ZHANG J Z, ZHANG H J, SHI L. Ecophysiological evaluation of the potential invasiveness of Rhus typhina in its non-native habitats [J]. Tree Physiology, 2009, 29(11): 1307. DOI: 10.1093/treephys/tpp065.
[35] WANG C Y, ZHOU J W, JIANG K, LIU J. Differences in leaf functional traits and allelopathic effects on seed germination and growth of Lactuca sativa between red and green leaves of Rhus typhina [J]. South African Journal of Botany, 2017, 111: 17-22. DOI: 10.1016/j.sajb.2017.03.019.
[36] FANG W S, MA C G. Jinzhou Yearbook [M]. Shenyang: Liaoning Nationality Publishing House, 2015: 59. (in Chinese)
[37] GONG X Q. Jinan Yearbook. [M]. Jinan: Jinan Publishing House, 2016: 34. (in Chinese)
[38] ZHANG W L, XU A G, ZHANG R L, JI H J. Review of soil classification and revision of China soil classification system [J]. Scientia Agricultura Sinica, 2014, 47: 3214-3230. (in Chinese)
[39] JIANG K, WU B D, WANG C Y, RAN Q. Ecotoxicological effects of metals with different concentrations and types on the morphological and physiological performance of wheat [J]. Ecotoxicology and Environmental Safety, 2019, 167: 345-353. DOI: 10.1016/j.ecoenv.2018.10.048.
[40] JEONG N, MOON J K, KIM H S, KIM C G, JEONG S C. Fine genetic mapping of the genomic region controlling leaflet shape and number of seeds per pod in the soybean [J]. Theoretical and Applied Genetics, 2011, 122(5): 865-874. DOI: 10.1007/s00122-010-1492-5.
[41] WANG Z, ZHANG L. Leaf shape alters the coefficients of leaf area estimation models for Saussurea stoliczkai in central Tibet [J]. Photosynthetica, 2012, 50(3): 337-342. DOI: 10.1007/s11099-012-0039-1.
[42] FORKMAN J. Estimator and tests for common coefficients of variation in normal distributions [J]. Communications in Statistics-Theory and Methods, 2009, 38(2): 233-251. DOI: 10.1080/03610920802187448.
[43] KRISHNAMOORTHY K, LEE M. Improved tests for the equality of normal coefficients of variation [J]. Computational Statistics, 2014, 29(1, 2): 215-232. DOI: 10.1007/s00180-013-0445-2.
[44] OLEJNIK S F, ALGINA J. Generalized eta and omega squared statistics: Measures of effect size for some common research designs [J]. Psychological Methods, 2003, 8(4): 434-447. DOI: 10.1037/1082-989X.8.4.434.
[45] BAKEMAN R. Recommended effect size statistics for repeated measures designs [J]. Behavior Research Methods, 2005, 37(3): 379-384. DOI: 10.3758/BF03192707.
[46] REEF R, LOVELOCK C E. Historical analysis of mangrove leaf traits throughout the 19th and 20th centuries reveals differential responses to increases in atmospheric CO2 [J]. Global Ecology and Biogeography, 2014, 23(11): 1209-1214. DOI: 10.1111/geb.12211.
[47] WRIGHT I J, REICH P B, CORNELISSEN J H C, FALSTER D S, GROOM P K, HIKOSAKA K, LEE W, LUSK C H, NIINEMETS U, OLEKSYN J, OSADA N, POORTER H, WARTON D I, WESTOBY M. Modulation of leaf economic traits and trait relationships by climate [J]. Global Ecology and Biogeography, 2005, 14(5): 411-421. DOI: 10.2307/3697523.
[48] de FRENNE P, GRAAE B J, RODRIGUEZ-SANCHEZ F, KOLB A, CHABRERIE O, DECOCQ G, de KORT H, de SCHRIJVER A, DIEKMANN M, ERIKSSON O, GRUWEZ R, HERMY M, LENOIR J, PLUE J, COOMES D A, VERHEYEN K. Latitudinal gradients as natural laboratories to infer species’ responses to temperature [J]. Journal of Ecology, 2013, 101(3): 784-795. DOI: 10.1111/1365-2745. 12074.
[49] XIAO H G, WANG C Y, LIU J, WANG L, DU D L. Insights into the differences in leaf functional traits of heterophyllous Syringa oblata under different light intensities [J]. Journal of Forestry Research, 2015, 26(3): 613-621. DOI: 10.1007/ s11676-015-0100-6.
[50] WANG C Y, LIU J, XIAO H G, ZHOU J W. Differences in leaf functional traits between Rhus typhina and native species [J]. CLEAN-Soil, Air, Water, 2016, 44(11): 1591-1597. DOI: 10.1002/clen.201600144.
[51] HULSHOF C M, VIOLLE C, SPASOJEVIC M J, MCGILL B, DAMSCHEN E, HARRISON S, ENQUIST B J. Intra-specific and inter-specific variation in specific leaf area reveal the importance of abiotic and biotic drivers of species diversity across elevation and latitude [J]. Journal of Vegetation Science, 2013, 24(5): 921-931. DOI: 10.1111/ jvs.12041.
(Edited by HE Yun-bin)
中文导读
入侵树种火炬树叶功能性状在中国北部的变异性
摘要:功能性状,特别是叶功能性状,是探究入侵植物成功入侵的核心问题。本研究旨在探究不同气候带(分为暖温带和寒温带)和入侵程度(分为低度入侵和高度入侵)的入侵树种火炬树叶功能性状及其变异性的差异。火炬树的入侵程度对其叶功能性状无显著影响。但是,高度入侵的火炬树叶的叶绿素含量和叶氮含量的变异性分别比低度入侵的火炬树高约66.023%和68.615%。暖温带火炬树叶的叶绿素含量和叶氮含量分别比寒温带火炬树的高约18.432%和16.337%。此外,暖温带火炬树的比叶面积的变异性比寒温带火炬树的高约59.802%。总之,火炬树叶的叶绿素含量和叶氮含量以及比叶面积及其变异性可能是火炬树在不同气候带成功入侵的关键生态策略。
关键词:气候区;入侵树种;变异系数;变异性;比叶面积;火炬树
Foundation item: Project(31300343) supported by the National Natural Science Foundation of China; Project(Y20160023) supported by Open Science Research Fund of State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, China; Project supported by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China; Project supported by Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment
Received date: 2018-12-07; Accepted date: 2019-09-03
Corresponding author: WANG Cong-yan, PhD, Associate Professor; Tel: +86-511-88790955; E-mail: liuyuexue623@163.com; ORCID: 0000-0002-6132-3319
Abstract: Functional traits, specifically leaf functional traits, are core-topics to explore importance to the invasion success of invasive plant species. This study aims to address the differences in leaf functional traits and their corresponding variability of the invasive tree staghorn sumac Rhus typhina L. with different invasion success, including lower and higher invasion success, in two climatic regions in North China, including a warm temperate region and a cold temperate region. No significant differences were found for leaf functional traits of staghorn sumac across different invasion success. However, the variability of leaf chlorophyll and nitrogen concentrations of staghorn sumac under higher invasion success were approximately 66.023% and 68.615% higher than those under lower invasion success, respectively. The leaf chlorophyll and nitrogen concentrations of staghorn sumac in the warm temperate region were approximately 18.432% and 16.337% higher than those in cold temperate region, respectively. The variability of specific leaf area of staghorn sumac in warm temperate region was approximately 59.802% higher than that in cold temperate region. Accordingly, leaf chlorophyll and N concentrations as well as specific leaf area of staghorn sumac and their corresponding variability may play an essential role in shaping ecological success of studied invader along a climatic gradient.


