Trans. Nonferrous Met. Soc. China 23(2013) 2229-2235
Interfacial reactions and bending strength of SiC/A356/FeNi50 composite fabricated by gas pressure infiltration
Chun-xue MA, Jia-kang YU, Chen XUE, Zhi-qing ZHANG
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 26 March 2012; accepted 8 June 2013
Abstract:
The SiC/A356/FeNi50 composite was fabricated by gas pressure infiltration. The interfacial region of the SiC/A356/FeNi50 composite consisted of FeNi50 reaction layer, Al reaction layer and Al alloy matrix. The main intermetallic compounds were (Fe,Ni)4(Al,Si)13 and (Fe,Ni)2(Al,Si)5 at the Al reaction layer and FeNi50 reaction layer, respectively. The bending behavior versus different infiltration temperatures and holding times was also investigated. The bending strength at 670 °C was the highest and close to the bending strength of Al alloy (223 MPa), and 46% of SiC/A356. The brittle intermetallic compounds existing at the interface induced the decreasing of the bending strength. The pores were reduced by adequate heating time due to the homogeneous temperature of preform, which was beneficial to improve the bending strength of the composite.
Key words:
infiltration; metal matrix composites; bending strength; interfacial reactions;
1 Introduction
Silicon carbide reinforced aluminum (SiC/Al) composites have received considerable attention in electronic packaging applications due to the attractive properties resulting from the combination of their constituents, such as high thermal conductivity, high specific strength, tailorable coefficient of thermal expansion and low density [1-3]. The properties of the composites are influenced by many factors, including the volume fraction of inclusions, interfacial bonding between inclusions and matrix, and the characteristics of matrix and inclusions itself. The volume fraction of the inclusion in the SiC/Al composite with a bimodal particle distribution is increased by distributing fine SiC particles in the interspace of coarse SiC particles in order to adjust the coefficient of thermal expansion of the composite [4]. The pressure infiltration and spark plasma sintering are two common ways used to fabricate the SiC/Al composite in the current works [5-7], and the interface condition of the composite fabricated by pressure infiltration seems more favorable while it still remains some problems by spark plasma sintering method. The studies on the thermal expansion [8,9], thermal conductivity [10,11] and mechanical properties [12,13] have also been carried out with the support of the experimental results and theoretical models. Thus, the researches of SiC/Al composites involved microstructure and properties in the recent ten years are quite comprehensive and deep.
However, most of the studies are about the SiC/Al composite itself. Actually, the SiC/Al composite should be jointed with the components of different materials, such as alloys, glasses or ceramics, to obtain the final products in practical applications. Due to the characters of inhomogeneous distribution and multi-phase in the SiC/Al composite, the bonding between the composite and metal components cannot be carried out effectively. The traditional welding methods, such as laser beam welding, make the SiC dissolve and produce a large number of interfacial reactions, which introduce Al4C3 and Al4SiC4 brittle phases to the jointing area, and will finally decrease the mechanical properties of the composites [14-16]. Therefore, this work which develops a new method to join SiC/Al composites with packaging components has important theoretical significance and practical value.
In this work a concurrent integration (CI) method was used to join the composite to the components of the alloys. The CI is a new method was used to join the composite with the alloy component by chemical reactions or other physical diffusion between the composite and alloys during the infiltration process, and the method can prevent the formation of the brittle phase and reduce the cost by about 50% [17]. The main components of the alloys are Ti alloy and FeNi alloy in the electronic market. The FeNi50 alloy was used in manufacturing frame and board for the integration circuit (IC), and thus the SiC/Al composites joining with the FeNi50 alloy by a CI method were fabricated. The chemical reactions in the joining area and the interface evolution were investigated, and the effects of infiltration parameters, such as temperature and holding time, on microstructure, interface and mechanical properties were also analyzed.
2 Experimental
Al-7Si-0.3Mg (A356) Al alloy was used as matrix. Green and abrasive grade SiC particles with a purity of 98.5% were used as inclusions. In order to obtain the high volume fraction of SiC particles, a bimodal particle size distribution of 5-50 μm in SiC particle sizes was adopted. The chemical composition of the FeNi50 alloy was listed in Table 1.
Table 1 Chemical composition of FeNi50 alloy (mass fraction, %)
Preparation of the compacts turned out to be one of the most delicate aspects of the experiments. In the case of mixtures the process required two stages: one was the mixing of the particles, and the other was the packing of the mixtures. Mixing was carried out in ethanol via the method described in Ref. [18]. The mixtures were then dried, introduced into the tube and packed as above. Strokes and vibrations on dry mixtures were identified as the main sources of segregation. In addition, other procedures were tried for the packing stage so as to reduce segregation and attain the maximum compactness possible. All reduced segregation (at least visually) decreased the maximum compactness at the same time. Here we described one of the procedures. The wetted mixture was introduced into the quartz tube, and the tube was vacuumized to partially evaporate the ethanol without fully drying the particles; subsequently, after applying 100 strokes, the compact was dried at a low temperature. After these procedures (if not specified, the results discussed here correspond to samples obtained through packing on dry mixtures), the mixtures of coarse and fine particles were prepared, and then were all packed in quartz tubes.
Infiltration was carried out in a pressure infiltration apparatus which was introduced in Refs. [19,20]. The Al alloy was placed in the lower furnace and the SiC/FeNi50 was placed in the upper furnace. The temperature of the lower furnace was set at 800 °C, and the temperatures of the upper furnace were set at 670, 690, 710 and 730 °C, respectively, for the different samples. Before heating, pressure of the furnace was vacuumized to 4000 Pa. Then the Al alloy was melted in the lower furnace with SiC preform and Fe-Ni50 alloy substrate being preheated in a steel cylinder attached in the upper furnace. After achieving the specified temperature, the system was allowed 2, 4, 6, 10, 14 and 18 min, respectively, to approach thermal equilibrium. Finally, the pressure for infiltration was applied by introducing air into pre-vacuumed chambers. Solidification of the metal alloy occurred within approximately 1 min after pressurizing the melt.
The microstructure of the composites was observed by scanning electron microscopy (SEM, Zeiss Supra 55) and optical microscopy (OM). XRD (PHILIPS X’Pert Pro) was used to analyze the composition of the microstructure. The 3 mm×4 mm×36 mm specimens were prepared for bending strength test which was carried out under a three-point loading using a Instron1195 machine. In all the cases the results represent the average of three tests.
3 Results and discussion
3.1 Interfacial reaction layer
The microstructure of the composites are greatly influenced by both temperature and holding time during heterogeneous bonding process. Figure 1 shows the microstructure of SiC/A356/FeNi50 at different infiltration temperatures for the holding time of 10 min.
The interface of SiC/A356 and FeNi50 can be clearly seen, which shows a great bonding between the two materials. The FeNi50, interfacial reaction layer (including reaction layer on the side of the FeNi50 substrate and the Al alloy), Al alloy matrix and the SiC/A356 are distributed from upper to down, respectively. The SiC/A356 composites at different infiltration temperatures are all dense and microscopically homogeneous, and seldom particle cluster is observed. The pores or cracks cannot be observed at the interfacial region until the infiltration temperature reaches 730 °C. A spindly crack with approximately 20 μm in length can be observed at the reaction layer. The thickness of the reaction layer on the side of the FeNi50 substrate keeps a constant as the temperature changes, while the thickness of the reaction layer on the side of Al alloy matrix has a quick increase up to 690 °C at first and then a nearly linear increase with the increasing of infiltration temperature, which is depicted in Fig. 2.
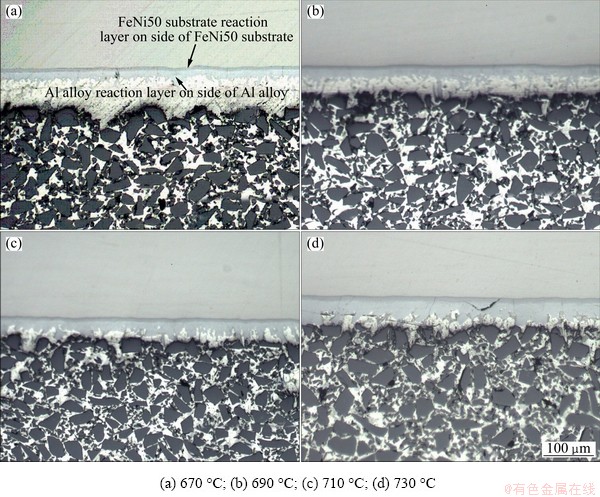
Fig. 1 Effect of infiltration bonding temperature on structure of SiC/Al/FeNi50 joints
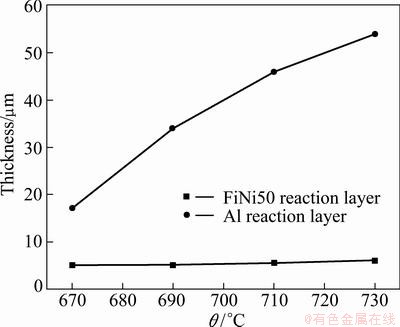
Fig. 2 Thickness of reaction layer versus infiltration temperature
The value of the thickness is measured via average ten figures in different regions and the errors are in the range of 5%-9%.
Furthermore, holding time is another parameter significantly affecting the interfacial bonding. Figure 3 shows the microstructure of SiC/A356/FeNi50 infiltrated at 710 °C for different holding time.
The thickness of the interfacial reaction layer varies a little as the holding time increases, which indicates that it is not influenced by the holding time but the temperature. However, the short holding time introduces pores at the interface, and the number and size of the pores decrease as the holding time increases. It can be seen from Figs. 3 (a) and (b) that the pores at the 2 min have a continuous distribution while the pores at the 6 min become finer. A well interfacial bonding and no pores can be observed at the 10 min. The pores will produce the stress concentration under loading and therefore they should be avoided in order to improve the bonding quality.
3.2 Interfacial reaction products
Figure 4 shows the microstructure of SiC/A356/ FeNi50 joints (P=1.2 MPa, θ=690 °C, t=10 min) which consisted of FeNi50 substrate, interfacial reaction layer and SiC/Al matrix from left to right, respectively.
Line analysis of chemical composition at the SiC/A356/FeNi50 interface is investigated and it is shown in Fig. 4. The length of the total line is 182 μm and the interface region is approximately in the range of 60-120 μm. The Si is mainly distributed at the SiC/Al side and seldom is observed at the FeNi50 region. The intensities of the Fe and Ni, which exhibit the very similar distributions, decrease from the FeNi50 side to the SiC/Al side at the interface. The Fe, Ni, Al and Si
keep constant at the interfacial region resulting from the existence of intermetallics which may influence significantly the mechanical properties of the composites [21]. Thus it is important to investigate the formation and growth of the intermetallics.
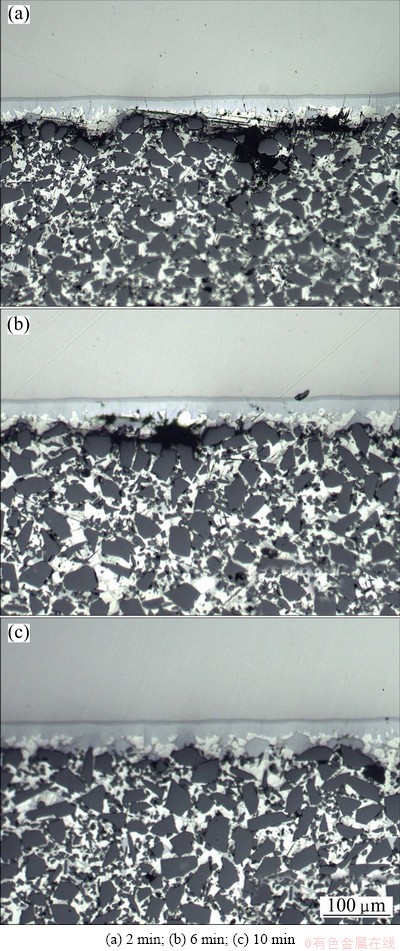
Fig. 3 Effect of holding time on microstructure of SiC/Al/ FeNi50 joints
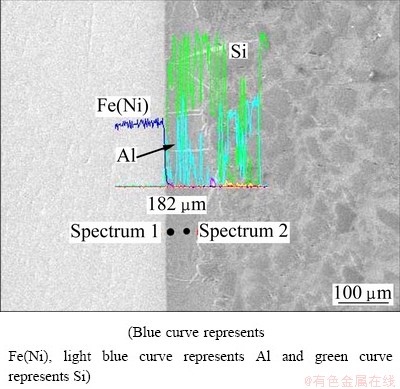
Fig. 4 Microstructure and line analysis of chemical composition at SiC/Al/FeNi50 interface
Figure 5 shows XRD pattern of fracture surface at infiltration temperature of 690 °C.
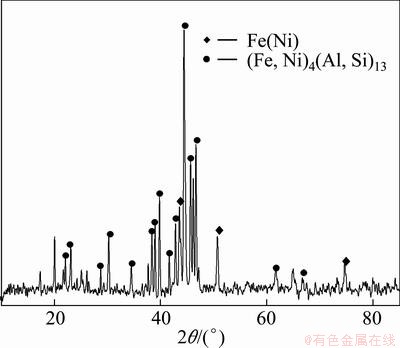
Fig. 5 XRD pattern of fracture surface on FeNi50 side
The results show that (Fe,Ni)4(Al,Si)13 and little Fe-Ni solid solution exist on the fracture surface. However, another intermetallic may exist at the FeNi50 reaction layer due to the different chemical composition at the Al reaction and FeNi50 reaction layers, which is detected by EDS analysis (Fig. (4)) and the results are shown in Table 2.
Table 2 EDS result of Al reaction and FeNi50 reaction region
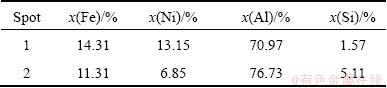
It can be deduced that the new intermetallic may be (Fe,Ni)2(Al,Si)5 because Fe2Al5 phase normally exists in the Fe-Al infiltration reaction as reported in many articles [22,23]. However, no direct evidence confirms this point and more work should be done further.
Figure 6 shows the interfacial reaction process of SiC/A356/FeNi50.
In the initial stage of infiltration, the Fe and Ni were dissolved and absorbed by molten Al. The chemical reactions carried out at the FeNi50 side (Fig. 6(b)). The thickness of reaction layer was only 3-4 μm which may be due to the existence of Si. The thickness of interfacial layer increased as a result of the diffusion of Al along the c axis by addition of Si which would be enriched at the interface. Meanwhile, the Al reaction layer begins to form (Fig. 6(c)). The Fe and Ni can diffuse continuously to the Al as a result of the high temperature in Al alloy. As the temperature decreased, the peripheral of the FeNi50 alloy firstly cooled down, a temperature gradient was generated between the Al and the FeNi50, and the vertical solidification of the (Fe,Ni)4(Al,Si)13 was generated in Al oriented columnar crystal along the Al-FeNi50 temperature gradient. The growing up behavior of the Al reaction layer can be seen in Fig. 6(d). In the later period of infiltration, the Al reaction layer grew continuously along the temperature gradient. In the initial stage of infiltration, (Fe,Ni)4(Al,Si)13 priority nucleated in the crystal defect and then grew up. Thus, the interfacial reaction products are shown to be a discontinuous distribution. Later, the interfacial reaction accelerated the growth of intermetallics and thus the thickness of the reaction layer increased.
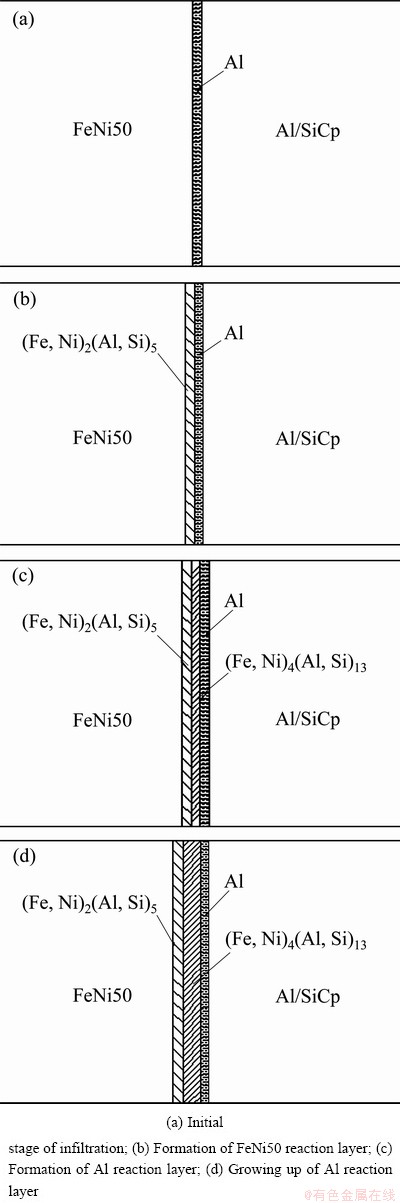
Fig. 6 Interfacial reaction process of SiC/Al/FeNi50
3.3 Bending strength
Figure 7 shows the bending strength of SiC/A356/FeNi50 joints contrast with SiC/A356 at different infiltration temperatures.
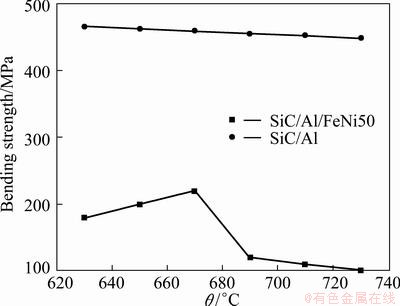
Fig. 7 Bending strength of SiC/Al/FeNi50 joints contrast with SiC/Al at different infiltration temperatures
The bending strength of the SiC/A356 composite keeps nearly constant as the infiltration temperature changes. Oppositely, the bending strength of the SiC/A356/FeNi50 is significantly influenced by the infiltration temperature. At 670 °C, low temperature induces poor diffusion as a result of thin interface (24.6 μm) and relatively good bending strength (215 MPa). The value of this bending strength is close to the bending strength of the Al alloy (223 MPa), and 46% of the SiC/A356 composite (467 MPa). When the temperature is lower, there is less or even no interfacial bonding which leads to a lower bending strength. Certain thickness of interfacial reaction layer helps the bonding of SiC, Al and FeNi50 which is not easy. As temperature increases, diffusivity of metals increases and thickness of interface increases which lead to a quickly decreasing of interfacial bending strength caused by brittleness of interfacial reaction layer. As temperature reaches 690 °C, bending strength decreases to 112 MPa, only 26% of the SiC/A356. After that, it decreases slowly as infiltration temperature increases.

Fig. 8 Relationship between bending strength of joints and heat holding time when infiltrated at 670 °C
In addition, holding time affects greatly the bending strength because the microstructure is sensitive to the holding time as is described in the discussion above. Figure 8 depicts the relationship between the bending strength of joints and holding time when infiltrated at 670 °C.
As holding time is lower than 10 min, bending strength is lower and it increases sharply as the holding time increases. The inhomogeneous temperatures in the preform, resulting from the insufficient holding time, lead to the imperfect infiltration and pores nearby the interface which reduce the bonding strength. A maximum bending strength (215 MPa) is obtained as holding time reaches 10 min. An adequate holding time makes preform heated homogeneously and reduces formation of pores, which are beneficial to improving bending strength of the composite. Furthermore, it is very interesting to observe that bending strength varies a little as holding time continuously increases. Thus, a minimum holding time is necessary to insure a homogeneous temperature in preform, which is beneficial to reducing the pores at the bonding region and obtaining a well infiltrated composite.
4 Conclusions
1) Higher infiltration temperature and lower holding time induce more cracks or pores at the bonding region. The thickness of reaction layer on side of FeNi50 keeps steadiness as temperature is changed, while thickness of reaction layer close to Al alloy matrix has a quickly increase up to 690 °C at first and then a nearly linear increase with the increasing of bonding temperature.
2) Interfacial region of SiC/A356/FeNi50 consists of reaction layer on side of FeNi50 substrate, reaction layer on side of Al alloy and Al alloy matrix. The main intermetallics at reaction layer is (Fe,Ni)4(Al,Si)13 on side of Al alloy. Interfacial reaction layer of the SiC/A356/FeNi50 composite comes from a process of formation and growth.
3) Bending strength decreases as the infiltration temperature increases. The value of bending strength at 670 °C is close to the bending strength of Al alloy (223 MPa), and 46% of SiC/A356. Brittle intermetallic compounds existing at interface induces the decreasing of bending strength. Adequate holding time makes preform temperature homogeneous and reduces the formation of pores, which is beneficial to improving bending strength of the composite.
References
[1] HUBER T, DEGISCHER H P, LEFRANC G, SCHMITT T. Thermal expansion studies on aluminium-matrix composites with different reinforcement architecture of SiC particles [J]. Composites Science and Technology, 2006, 66: 2206-2217.
[2] ZHU Xiao-min, YU Jia-kang, WANG Xin-yu. Microstructure and properties of Al/SiC/Si composites for electronic packaging [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(7): 1686-1692.
[3] MOLINA J M, PRIETO R, NARCISO J, LOUIS E. The effect of porosity on the thermal conductivity of Al-12wt.% Si/SiC composites [J]. Scripta Materialia, 2009, 60: 582-585.
[4] MOLINA J M., SARAVANAN R A, ARPON R. Pressure infiltration of liquid aluminium into packed SiC particulate with a bimodal size distribution [J]. Acta Materialia, 2002, 50: 247-257.
[5] REN S B, QU X H, GUO J, HE X B, QIN M L, SHEN X Y. Net-shape forming and properties of high volume fraction SiCp/Al composites [J]. Journal of Alloys and Compounds, 2009, 484: 256-262.
[6] CHU K, JIA C C, TIAN W H, LIANG X B, CHEN H, GUO H. Thermal conductivity of spark plasma sintering consolidated SiCp/Al composites containing pores: Numerical study and experimental validation [J]. Composites: Part A, 2010, 41: 161-167.
[7] ZHANG Z H, WANG F C, LUO J, LEE S K, WANG L. Microstructures and mechanical properties of spark plasma sintered Al-SiC composites containing high volume fraction of SiC [J]. Materials Science and Engineering A, 2010, 527: 7235-7240.
[8] NAM T H, REQUENA G, DEGISCHER P. Thermal expansion behavior of aluminum matrix composites with densely packed SiC particles [J]. Composites: Part A, 2008, 39: 856-865.
[9] ZHANG Q, WU G H, JIANG L T, CHEN G Q. Thermal expansion and dimensional stability of Al-Si matrix composite reinforced with high content SiC [J]. Materials Chemistry and Physics, 2003, 82: 780-785.
[10] CHU K, JIA C C, LIANG X B, CHEN H, GUO H. The thermal conductivity of pressure infiltrated SiCp/Al composites with various size distributions: experimental study and modeling [J]. Materials and Design, 2009, 30: 3497-3503.
[11] MOLINA J M, NARCISO J, WEBER L. Thermal conductivity of Al-SiC composites with monomodal and bimodal particle size distribution [J]. Materials Science and Engineering A, 2008, 480: 483-488.
[12] OZBEN T, KILICKAP E, CAKIR O. Investigation of mechanical and machinability properties of SiC particle reinforced Al-MMC [J]. Journal of Materials Processing Technology, 2008, 198: 220-225.
[13] KAYNAK C, BOYLU S. Effects of SiC particulates on the fatigue behaviour of an Al-alloy matrix composite [J]. Materials and Design, 2006, 27: 776-782.
[14] LIENERT T J, BRANDON E D, LIPPOLD J C. Laser and electron beam welding of SiCp reinforced aluminum A-356 metal matrix composites [J]. Scripta Metallurgica and Materialia, 1993, 28: 1341-1346.
[15] HUANG R Y, CHEN S C, HUANG J C. Electron and laser beam welding of high strain rate superplastic Al-6061/SiC composites [J]. Metallurgical and Materials Transactions A, 2001, 32: 2575-2584.
[16] ELLIS M D B. Joining of aluminium based metal matrix composites [J]. International Materials Reviews, 1996, 41: 41-58.
[17] ADAMS R W, NOVICH B E, FENNESSY K P. Concurrent IntegrationTM of Al/SiC MMIC Packages [C]//Proceedings of the 1995 International Symposium on Microelectronics, ISHM. Los Angeles CA, 1995: 36-41.
[18] LEE J H, LACKEY W J, BENZEL J F. Ternary packing of SiC and diamond particles in ethanol [J]. Journal of Materials Research, 1996, 11: 2804-2810.
[19] YU W, YU J K. Silicon dissolution and interfacial characteristics of Si/Al composites fabricated by gas pressure infiltration [J]. Materials Chemistry and Physics, http://dx.doi.org/10.1016/j.matchemphys. 2013.02.032.
[20] XUE C, YU J K. Enhanced thermal conductivity in diamond/ aluminum composites: Comparison between the methods of adding Ti into Al matrix and coating Ti onto diamond surface [J]. Surface and Coatings Technology, 2013, 217: 46-50.
[21] SURESH S, MORTENSEN A, NEEDLEMAN A. Fundamentals of Metal-Matrix Composites [M]. UK:Butterworth-Heinemann, 1993, 233-250.
[22] SU C W, LEE J W, WANG C S, CHAO C G, LIU T F. The effect of hot-dipped aluminum coatings on Fe-8Al-30Mn-0.8C alloy [J]. Surface and Coatings Technology, 2008, 202: 1850-1852.
[23] POCHEC E, JOZWIAK S, KARCZEWSKI K, BOJER Z. Fe-Al phase formation around SHS reactions under isothermal conditions [J]. Journal of Alloys and Compounds, 2011, 509: 1124-1128.
气压浸渗法制备SiC/A356/FeNi50复合材料的界面反应和抗弯强度
马春雪,于家康,薛 晨,张志庆
西北工业大学 凝固技术国家重点实验室,西安 710072
摘 要:采用气压浸渗法制备SiC/A356/FeNi50复合材料。SiC/A356/FeNi50的界面由FeNi50反应层、Al反应层和Al合金基体组成。(Fe,Ni)4(Al,Si)13和 (Fe,Ni)2(Al,Si)5 作为主要的界面反应产物分别存在于FeNi50反应层和Al反应层中。讨论了不同浸渗温度和保温时间下的材料弯曲行为。结果表明:670 °C时的抗弯强度是所有温度下的最好的,与Al合金的(223 MPa)接近,并达到了SiC/A356抗弯强度的46%。由于界面中脆性相的存在而导致抗弯强度的下降。合适的保温时间能使预制体温度更加均匀,减少空洞的形成,这对提高复合材料的抗弯强度具有重要意义。
关键词:浸渗;金属基复合材料;抗弯强度;界面反应
(Edited by Chao WANG)
Foundation item: Project (60776019) supported by the National Natural Science Foundation of China; Project (61-TP-2010) supported by Research Fund of the State Key Laboratory of Solidification Processing (NWPU), China
Corresponding author: Chun-xue MA; Tel: +86-29-88494987; E-mail: jkyu@nwpu.edu.cn
DOI: 10.1016/S1003-6326(13)62722-3
Abstract: The SiC/A356/FeNi50 composite was fabricated by gas pressure infiltration. The interfacial region of the SiC/A356/FeNi50 composite consisted of FeNi50 reaction layer, Al reaction layer and Al alloy matrix. The main intermetallic compounds were (Fe,Ni)4(Al,Si)13 and (Fe,Ni)2(Al,Si)5 at the Al reaction layer and FeNi50 reaction layer, respectively. The bending behavior versus different infiltration temperatures and holding times was also investigated. The bending strength at 670 °C was the highest and close to the bending strength of Al alloy (223 MPa), and 46% of SiC/A356. The brittle intermetallic compounds existing at the interface induced the decreasing of the bending strength. The pores were reduced by adequate heating time due to the homogeneous temperature of preform, which was beneficial to improve the bending strength of the composite.


