J. Cent. South Univ. (2012) 19: 331-339
DOI: 10.1007/s11771-012-1009-2![]()
Magnetic iron oxide nanoparticles carrying PTEN gene to reverse cisplatin-resistance of A549/CDDP cell lines
MIN Ling-feng(闵凌峰), HE Ling-ling(何玲玲), CHEN Qiong(陈琼), YU Qiao(俞巧), XIE Ming-xuan(谢明萱)
Department of Geriatric Medicine, Department of Respiratory, Xiangya Hospital of Central South University,Changsha 410008, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract:
To evaluate the feasibility of using magnetic iron oxide nanoparticle as wild PTEN gene carrier for transfection in vitro to reverse cisplatin-resistance of A549/CDDP cells, A549/CDDP cells were transfected with the wild PTEN gene expression plasmid (pGFP-PTEN) by magnetic iron nanoparticle and lipo2000. The transfection efficiency was detected by fluorescence microscope and flow cytometer. The expression levels of PTEN mRNA and protein were detected by reverse transcription polymerase chain reaction (RT-PCR) and immunocytochemistry analysis. The effect of PTEN transfection on cell cycle enhances the sensitivity of A549/CDDP to cisplatin and nanoparticle-mediated transfection has a higher efficiency than that of the liposome-mediated group. The apoptosis level was up-regulated in PTEN transfection group. The magnetic iron oxide nanoparticle could be used as one of the ideal gene carriers for PTEN gene delivery in vitro. PTEN can be an effective target for reversing cisplatin-resistance in lung cancer.
Key words:
magnetic iron oxide nanoparticle; liposome transfection; lung cancer; PTEN; cisplatin-resistance;
1 Introduction
Drug resistance in lung cancer patients is an important reason for the failure of chemotherapy and leads to bad prognosis. In recent years, a lot of drugs were ascertained that could reverse drug resistance of cancers, such as cyclosporine A, verapamil and quinidine. However, it is the main obstacle in clinical application that these drugs have a low reversal activity of drug resistance, as well as high toxicity. With the development of research, a number of genes related to the mechanism of drug resistance in lung cancer were found, which promoted gene-targeted therapy to be a hot spot in tumor study.
PTEN is a tumor suppressor which was found in 1997 located on 10q23.3 [1-2]. Importantly, it is the second-most frequently mutated gene in human cancer after p53, and its inactivity is implicated in development of several cancers [3]. It is reported that mutation, homozygous deletion and methylation lead to PTEN inactivation [4]. Notably, PTEN protein expression is closely correlated with the pathological grade, prognosis, invasion and metastasis of tumor [5-7]. And the cell growth is inhibited after transfecting PTEN into the tumor. In particular, recent researches implied that PTEN was closely related with the sensitivity to chemotherapeutic drugs in cancers [8]. TANAKA et al [9] transplanted PTEN into Docetaxel-resistant bladder cancer cell lines, and found that there was an increase in sensitivity to Docetaxel in bladder cancer. ZHOU et al [10] transfected PTEN into acute lymphoblastic leukemia cell lines, and also discovered that the sensitivity to doxorubicin increased. However, so far, there are few relevant studies about the relation between PTEN and drug-resistance in lung cancer.
Gene transfection is a main technology of gene treatment, and liposome-mediated transfection is one of the classical methods. However, liposome-mediated transfection leads to cell toxicity. Besides, it takes part in cellular physiological activities, brings the up-regulation or down-regulation of gene expression and causes error valuation of efficiency [11]. In response to these shortcomings, many studies have reported about the feasibility of bio-degradable nanoparticles or microparticles as carriers mediating gene transfection in recent years [12]. But, there were no relevant in vitro reports on using nanoparticle to meditate PTEN transfection into A549/CDDP to reverse the drug resistance of lung cancer. Herein, PTEN expression plasmid was transfected into A549/CDDP by using liposome and nannoparticle as gene carriers, respectively, and a comparison was made between liposome carrier and nanoparticle carrier in transfection to evaluate the feasibility of nanoparticle as carrier of plasmid with PTEN expression transfection and to find out the efficiency to reverse the cisplatin-resistance in A549/CDDP and study the potential relationship between PTEN and lung cancer.
2 Experimental
2.1 Materials
2.1.1 Cells and plasmids
Human lung cancer cell lines (A549) and human cisplatin-resistant lung cancer cells (A549/CDDP) were purchased from Cell Center in Xiangya Hospital of Central South University, Changsha, China. The plasmid with wild PTEN gene (pGFP-PTEN) and its control plasmid (pGFP) were gifts from Doctor Kenneth M. Yamada of U.S. Nation Health Institutes of Health. These two plasmids both contained green fluorescent protein (GFP).
2.1.2 Reagents
Restrictive endonuclease Hind III and Xbal were purchased from BioLabs Company. Magnetic iron oxide nanoparticles (polyMAG-1000) were obtained from Chemicell GmbH Company. Liposome (Lipofectamine 2000) was from Invitrogen Company. Reverse transcription kits were purchased from Promega Company. Large extraction kits of plasmid (Tip500) were from Macherey-nagel Company. Mouse anti-human PTEN polyclonal antibodies were obtained from Beijing Biosynthesis Biotechnology Company. SP ultra-sensitive immunohistochemical kits were from Beijing Zhongshan Goldbridge Biotechnology Company.
2.2 Methods
2.2.1 Plasmid preparation
Two kinds of plasmids were transfected into competent bacteria and the positive clones were chosen to enlarge the culture, then the plasmids were drew out, digested by the endonuclease Hind III and Xba I and qualified by sequencing. After identifying the required plasmids, a large number of plasmids were produced by following Tip500 instructions from Macherey-nagel Company.
2.2.2 Cell culture
A549/CDDP was grown in DMEM supplemented with 10% calf serum at 37 °C containing 5% CO2, and the concentration of DDP was maintained at 6 μmol to keep the cisplatin-resistance of A549/CDDP and the DMEM medium was changed two or three days once.
2.2.3 Gene transfection in vitro
A549/CDDP was seeded in a six-well plate at 5×104 per well, then the cells were washed twice with DMEM without serum 24 h later. For untransfected group (control) and empty vector group, plasmid without PTEN (pGFP) was mixed with polyMAG-1000 for 15 min at room temperature on the magnetic field before transfection, the concentrations of polyMAG-1000 and pGFP were both 8 mg/L. For group of Liposome- mediated transfection (Liposome group), transfection was followed by instruction and the concentrations of Lipofectamine 2000 and plasmid were 16 mol/L and 8 mg/L, respectively. For group of the magnetic iron oxide nanoparticle-mediated transfection (Nano group), plasmid with PTEN (pGFP-PTEN) was mixed with polyMAG-1000 for 15 min at room temperature in the magnetic field before transfection, and the concentrations of polyMAG-1000 and pGFP were both 8 mg/L. Media in the four groups were changed to DMEM supplemented with 10% calf serum 4 h after transfection. The transfection efficiencies of the four groups were detected by fluorescence microscope 24 h after transfection and the efficiencies were assessed by GFP expression tested by flow cytometer 48 h after transfection.
2.2.4 PTEN mRNA expression assay by RT-PCR
Total mRNA was extracted and transcribed into cDNA 1 d, 3 d, 5 d after transfection, respectively. cDNA was used as a template for PCR amplification to detect PTEN mRNA expression. The upstream primer of PTEN is 5′-GTAAGGACCAGAGACAAAAAG-3′, the downstream primer is 5′-CTTTTTTAGCATCTTGTT- CTG-3'; the upstream primer of β-actin is 5'-TGAAGT- GTGACGTGCATC-3', the downstream primer was 5'-GGAGGAGCAATGATCTTGAT-3'. All amplification products were separated in 2% agar gel electrophoresis and the amplified bands were scanned by the gel digital imaging system.
2.2.5 PTEN protein expression assay by immunocyto- chemistry
A549/CDDP was grouped as same as Section 2.2.3 after cell climbing film. A549/CDDP was washed with cold PBS three times 5 d after transfection and fixed in paraformaldehyde for 30 min. 50 μL DAB was added into A549/CDDP by following the instructions of SP ultra-sensitive immunohistochemical kits, then the cells were inoculated at room temperature for 5 min, and at the same time observed with microscope. These reactions were terminated by flushing with running water as soon as the positive signs were monitored on the microscope. Hematoxylin dyeing, alcohol dehydration, xylene transparencizing twice and neutral cover slipping were performed as described. Microscope was used to observe and pick up image. The criterion was that the yellow-dyed cells were positive cells. Positive cells were analysed with Motic medical imaging analysis system. The general process was that choosing ten fields in 10×40 highpower lens randomly in one specimen and then calculating the optical density in every field. The integral optical density of the specimen was the average optical density of the ten fields.
2.2.6 Effect of PTEN transfection on A549/CDDP cell growth
A549/CDDP was seeded in 96-well plate at 1×104 per well and each well contained 100 μL, then the cells were washed twice with DMEM without serum 24 h later and grouped as same as Section 2.2.3. Each group had four double wells. Cell survival ratio was detected by MTT 1, 2, 3, 4, 5 and 6 d after transfection, respectively. Cell survival ratio was equal to the absorbance in transfected group divided by the absorbance in untransfected group. The survival ratio curve was plotted with the number of days as the horizontal axis and the survival ratio as the vertical axis using data obtained previously.
2.2.7 Cisplatin-resistant index of A549/CDDP test by MTT
Exponential phase A549 and A549/CDDP were seeded in 96-well plate at 1×104 per well and each well contained 100 μL. Then, the cells were washed twice with DMEM without serum 24 h later. Experimental groups were (each group had four double wells): 1) A549 group: equal volume of DMEM was added; 2) A549/ CDDP group; 3) pGFP transfection group; 4) Group of Liposome-mediated transfection (Liposome group); 5) Group of the magnetic iron oxide nanoparticle-mediated (Nano group). Cisplatin was added into all groups at seven different concentrations of 0, 10, 20, 40, 80, 160 and 320 μmol/L. The survival ratio was tested by MTT and IC50 of cisplatin was analysed by Probit Regression Analysis in SPSS 24 h after cell culture. Cell survival ratio is equal to the absorbance in test group divided by the absorbance in control. Resistant index is equal to IC50 in test group divided by IC50 in A549.
2.2.8 Cell cycle and apoptosis assay by flow cytometer
Cells were collected 5 d after transfection and digested by 0.25% trypsin. DMEM supplemented with 10% fetal calf serum was used to terminate the digestion, and then 3 000 r/min centrifuged for 5 min and abandoning supernatant were performed. The cells were washed twice with PBS, 3 000 r/min centrifuged for 5 min and discarding supernatant were also performed. Then, the cells were fixed in 4 °C pre-cooling 70% cold ethanol, and 10 μL PI (100 μg/mL) and 10 μL Rnase (50 μg/mL) were added into the cells. After that, the cells were laid in 37 °C aqueous bath for 30 min in dark. Cell cycle and apoptosis were assayed by flow cytometer.
2.2.9 Cell toxicity test by MTT
A549/CDDP was seeded in 96-well plate at 1×104 per well and each well contained 100 μL. The plate was divided into two groups, polyMAG-1000 (2 μL/mL) and Lipofactamine 2000 (4 μg/mL) were added into the groups, respectively, and each group had four double wells. The cells were washed twice with PBS 4 h later, and 100 μL DMEM was added into the well to culture the cells for 20 h. MTT was added into the medium, and DMSO was added to detect the absorbance of each well with a 490 nm wavelength 4 h later.
2.2.10 Statistic analysis
Statistical analysis of experimental data was performed using the statistical package for social science software (SPSS for Windows, version 13.0). Data were expressed by average![]() standard deviation (S.D). Comparisons among different groups in different time were analysed by multivariate ANOVA. Comparisons between two groups were analysed by SNK-q test. IC50 was calculated by probit regression analysis. Significance is defined as P<0.05.
standard deviation (S.D). Comparisons among different groups in different time were analysed by multivariate ANOVA. Comparisons between two groups were analysed by SNK-q test. IC50 was calculated by probit regression analysis. Significance is defined as P<0.05.
3 Results
3.1 pGFP-PTEN
The pGFP-PTEN (6.7 kb) was digested by restrictive endonuclease Hind III and Xbal into pGFP (5.4 kb) and the inserted PTEN gene (1.3 kb). The sizes of electrophoresis bands in Fig. 1 are as the same as the sizes mentioned above, and no mutation and impairing are found in the subsequent sequencing, indicating that the sequence of pGFP-PTEN is right.
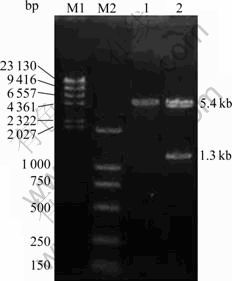
Fig. 1 Results of digestion of recombinant pGFP-PTEN: M1— λ-Hind III DNA marker; M2—DL2000 DNA marker; 1— Digestion product of pGFP; 2—Digestion product of pGFP- PTEN
3.2 Comparison of transfection efficiencies of liposome and magnetic iron oxide nanoparticle
Several fluorescent particles were found in cytoplasm under fluorescence microscope 48 h after transfection. Efficiencies were assessed by GFP expression detected by flow cytometer. The efficiencies of liposome-mediated transfection and magnetic iron oxide nanoparticle-mediated transfection are (32.15± 3.14)% and (52.22±2.41)%, respectively, and the later was apparently higher than the former (P>0.05). The data imply that the liposome and nanoparticle could transfect the target gene into cells and the latter transfection efficiency is higher than the former (Fig. 2).

Fig. 2 Efficiencies of liposome-mediated transfection and magnetic iron oxide nanoparticle-mediated transfection: (a) Fluorescent image in liposome-mediated transfection; (b) Fluorescent image in magnetic iron oxide nanoparticle- mediated transfection; (c) Bar chart depicting comparison of transfection efficiency between liposome-mediated transfection and nanoparticle-mediated transfection
3.3 PTEN mRNA expression level
PTEN mRNA expression level was monitored 1, 3 and 5 d after transfection, respectively. The expression level in pGFP-PTEN transfected groups is significantly higher than untransfected group and empty vector group. The difference of PTEN mRNA expression between nanoparicle-mediated transfection group and liposome- mediated transfection group 1 d after transfection is not statistically significant (P=0.07), but PTEN mRNA expression in nanoparticle-mediated transfection is higher than that in liposome-mediated transfection 3 d and 5 d after transfection (P<0.05). Furthermore, PTEN mRNA expression in liposome-mediated transfection 5 d after transfection is apparently lower than that 1 d and 3 d after transfection, while in nanoparticle-mediated transfection there are no differences among 1 d, 3 d and 5 d after transfection (Fig. 3).
3.4 PTEN protein expression level
PTEN protein expression level was detected by immunocytochemistry 5 d after PTEN transfection. PTEN protein expression is relatively weak in untransfected group (77.3±10.0) and empty vector group (81.1±16.8); while the expression level in pGFP-PTEN transfected group is apparently higher than the former two groups, and the expression in the nanoparticle- mediated transfection (305.7±63.4) is significantly higher than that in the liposome-mediated transfection (171.9±41.9) (P<0.05) (Fig. 4).
3.5 Effect of PTEN transfection on A549/CDDP cell growth
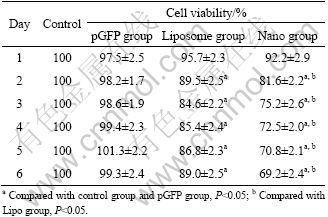
The cell viability of A549/CDDP cells in different groups was detected by MTT to calculate the inhibition ratio daily for 6 d after transfection. The survival ratio in the pGFP-PTEN transfected A549/CDDP has a downward trend after transfection compared with the empty vector group, but the trend is not statistically significant, and the survival ratio has a dramatic decline in transfected groups 2-6 d after transfection. Furthermore, the survival ratio in nanoparticle-mediated transfection is apparently lower than that in liposome- mediated transfection at the same day (Table 1).
3.6 Cell cycle and apoptosis
Statistical analysis shows that the number of cells in G0-G1 phase and the number of apoptotic cells are both significantly higher in group transfected with wild PTEN than those in other three groups (P<0.05) (Fig. 5 and Table 2).
3.7 Impact of PTEN transfection on cisplatin- resistance of A549/CDDP
Cisplatin-resistance of A549/CDDP in different groups was determined by MTT assay. IC50 in control group has no difference with that in empty vector group. It declines in transfected groups, and the decline is more significant in nano group than in liposome group (Table 3).

Fig. 3 Expression of PTEN mRNA after pGFP-PTEN transfection in A549/CDDP for different time (M—DL 2000 DNA marker; 1—Untransfected group; 2—Empty vector group; 3—Liposome-mediated transfection group; 4—Nanoparticle-mediated transfection group): (a) 1 d; (b) 3 d; (c) 5 d; (d): Curves depicting expression of PTEN mRNA
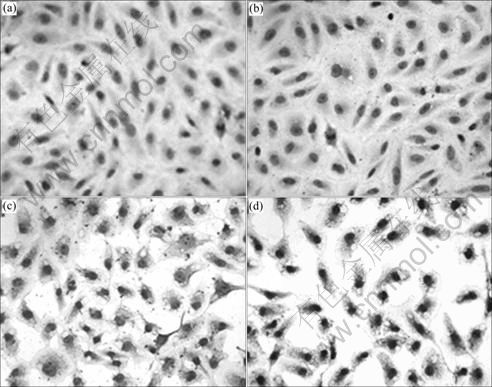
Fig. 4 PTEN protein expression level detected by immunocytochemistry in A549/CDDP cells after different treatments: (a) Untransfected group; (b) Empty vector group; (c) Group of liposome-mediated wild PTEN transfection; (d) Group of magnetic iron oxide nanoparticle-mediated wild PTEN transfection
Table 1 Cell viability of A549/CDDP after transfection
3.8 Cell toxicity of magnetic iron oxide nanoparticle
MTT assay was used to measure the absorbance of the cells to detect cell toxicity of magnetic iron oxide nanoparticle. The differences of absorbance in cells between polyMAG-1000 or Lipofactamine and in control group have no statistical significance at the experimental concentration and time. These analyses show that there is no apparent cell toxicity of ployMAG-1000 as a gene carrier at the experimental concentration and time.
4 Discussion
A main problem in gene therapy is how to make the target gene penetrate the cell membrane effectively. So far, the widely used carrier is liposome, but liposome has cell toxicity to some extent since it takes part in PKC signaling pathway and inhibits the activity of ATPase. In recent years, magnetic nano material as a modern carrier makes safe gene transfection possible. The magnetic iron oxide nanoparticle can bring target gene into cells with the help of photo grain, and can lead effective and safe target treatment in the magnetic field [13]. The polyMAG-1000 used in this work consists of magnetic iron oxide nanoparticle as the core and cationic polymer polyethyleneimine (PEI) as the cover. This special structure enhances the efficiency of transfection, combines closely with DNA, protects plasmid from degeneration of nuclease, and helps plasmid effuse from liposome and entry into nucleus. It is reported that the specific superparamagnetism in magnetic iron oxide nanoparticle can increase 5-10 folds of transfection efficiency in external magnetic field [14]. In this work, the transfection efficiency in magnetic iron oxide nanoparticle group ((52.22±2.41)%) is apparently higher than that in liposome group ((32.15±3.13)%) (P<0.05). Moreover, PTEN mRNA expression is higher in transfected groups than in untransfected group and control group, and the expression in nano group is apparently higher than in liposome group 1, 3 and 5 d after transfection. As the transfection time increases, PTEN mRNA expression in liposome group declines gradually, but the decline is not statistically significant until the fifth day; while the expression level in nano group does not decline obviously. The data mentioned above imply that magnetic iron oxide nanoparticle- mediated transfection is better than liposome-mediated transfection in maintaining the persistence of PTEN gene expression. In all, polyMAG-1000 as a gene carrier is feasible and superior to the liposome.

Fig. 5 Flow cytometer analysis results of cell cycle and apoptosis: (a) Control group; (b) Empty vector group; (c) Liposome group; (d) Nano group
Table 2 Flow cytometer analysis results of cell cycle and apoptosis (%)

Table 3 Impact of PTEN transfection on cisplatin-resistance of A549/CDDP (n=3)

PTEN is a tumor suppressor with dual-phosphatase activity that plays an essential factor in embryonic development, regulates cell cycle and apoptosis, inhibits metastasis and invasion of tumor cells, and induces the sensitivity to chemotherapy. Exogenous wild PTEN transfection can suppress tumor cell growth in gliomas, endometrial cancer, ovarian cancer, prostate cancer, breast cancer and melanoma [15-18]. In this work, it is found that the survival ratio of A549/CDDP cells declines 2-6 d after transfection, and it is apparently lower in nanoparticle-mediated transfection than in liposome- mediated transfection (P<0.05), suggesting that PTEN transfection could inhibit A549/CDDP cell growth and the inhibition is stronger in nanoparticle-mediated transfection than in liposome-mediated transfection. In the study of glioma, LI and SUN [19] found that wild PTEN transfection worked as tumor suppressor only in PTEN deficient cells, while it did not work in endogenous cells with PTEN expression. However, in the study of breast cancer and thyroid cancer, WANG et al [20] and BRUNI et al [21] discovered that wild PTEN transfection inhibited cells growth whether endogenous PTEN expressed or not. Moreover, the inhibition was stronger in PTEN deficient cells than endogenous PTEN expression cells. Differently, KNOBLOCH et al concluded that PI3K as a positive regulator and PTEN as a negative regulator in AKT/PKB pathway regulated cell growth and proliferation to maintain a dynamic equilibrium, either the enhancement of PI3K or the weakening of PTEN would break the balance, leading to carcinogenesis. The exogenous PTEN transfection could restore this balance to inhibit tumor cell growth and proliferation [22]. Fortunately, in this work, it is found that although there are PTEN mRNA and protein expression in A549/CDDP, A549/CDDP cell growth is inhibited by exogenous PTEN transfection, which supports the theory of KNOBLOCH et al. Besides, it is discovered that A549/CDDP cell growth inhibition by PTEN may be related to stasis of G1 phase and apoptosis.
In the respect of sensitivity to cisplatin in A549/ CDDP, IC50 is (193.8±21.3) μmol/L in A549/CDDP, and the resistant index is 11.5. IC50 is (120.3±11.5) μmol/L 72 h after transfection in liposome-mediated wild PTEN transfected A549/CDDP cells, and the resistant index is 7.1. IC50 is (72.8±10.9) μmol/L 72 h after transfection in nanoparticle-mediated wild PTEN transfected A549/CDDP cells, and the resistant index is 4.3. The data mentioned above imply that the wild PTEN transfection enhances the sensitivity to cisplatin in A549/CDDP and it is more sensitive in nanoparticle- mediated transfection than in liposome-mediated transfection. TANAKA et al transplanted PTEN into Docetaxel resistant bladder cancer cell lines, and found that there was an increase of sensitivity to Docetaxel in bladder cancer [9]. WU et al transplanted PTEN into cisplatin-resistant breast cancer cell lines, and found that there was an increase of sensitivity to cisplatin in breast cancer [15]. In this work, it is initially proved that PTEN is closely related to drug-resistant lung cancer, and PTEN transfection could enhance the sensitivity to chemotherapy in drug-resistant lung cancer.
In the respect of safety of magnetic iron oxide nanoparticle, it is found that there is no apparent cell toxicity in polyMAG-1000 at the experimental concentration and time. The response dose of polyMAG-1000, which has no cell toxicity and mutagenic effect, is greater than the recommended dose in vivo of magnetic iron oxide nanoparticle, e.g. Fe2O3 and Fe3O4. But cell toxicity occurs in PEI, which is due to the affinity and adhesion with cell membrane and related to the diameter, concentration and effect time of nanoparticle. In other words, a successful transfection should contain enough PTEN transfected into A549/CDDP and little cell damage or death. THOMAS and KLIBANOV [23] found that there was a linear increase of cell toxicity when PEI was over 24 ku. SOSCHER found that when PEI was over 7 μg/mL, it led to cell death [24]. In the research of combi- MAG1000, KROTZ et al [25] discovered that there were 15%-20% cell death at 1 μg/mL concentration for 4 h and 22% cell death for 24 h. In this work, high transfection efficiency and no apparent cell death at 2 μg/mL concentration are found.
According to this work, it is found that PTEN gene inhibits cell growth and proliferation by blocking G1 phase and inducing apoptosis, up-regulates the sensitivity to cisplatin in A549/CDDP, and reverses the drug resistance in lung cancer. Furthermore, it is realized that magnetic iron oxide nanoparticle is superior to liposome as a gene carrier, which provides new ideas to ameliorate the therapy of drug-resistant lung cancer.
5 Conclusions
1) The magnetic iron oxide nanoparticle-mediated PTEN transfection has higher efficiency and more PTEN expression in mRNA and protein than the liposome-mediated transfection. The magnetic iron oxide nanoparticle has no apparent cell toxicity as a gene carrier at the experimental concentration and time. The magnetic iron oxide nanoparticle (polyMAG-1000) could be used as one of the ideal gene carriers for gene delivery in vitro.
2) After PTEN is transfected into the A549/CDDP, the A549/CDDP cell growth slows down, the number of cells in G0-G1 phase and apoptotic cells both significantly become higher, and the sensitivity of A549/CDDP to cisplatin is enhanced. PTEN can be an effective target for reversing cisplatin-resistance in lung cancer.
References
[1] STECK P A, PERSHOUSE M A, JASSER S A, YUNG W K, LIN H, LIGON A H, LANGFORD L A, BAUMGARD M L, HATTIER T, DAVIS T, FRYE C, HU R, SWEDLUND B, TENG D H, TAVTIGIAN S V. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers [J]. Nat Genet, 1997, 15(4): 356-362.
[2] PLANCHON S M, WAITE K A, ENG C. The nuclear affairs of PTEN [J]. J Cell Sci, 2008, 121(Pt 3): 249-253.
[3] HO C M, LIN M C, HUANG S H, HUANG C J, LAI H C, CHIEN T Y, CHANG S F. PTEN promoter methylation and LOH of 10q22-23 locus in PTEN expression of ovarian clear cell adenocarcinomas [J]. Gynecol Oncol, 2009, 112(2): 307-313.
[4] IWANAGA K, YANG Y, RASO M G, MA L, HANNA A E, THILAGANATHAN N, MOGHADDAM S, EVANS C M, LI H, CAI W W, SATO M, MINNA J D, WU H, CREIGHTON C J, DEMAYO F J, WISTUBA I I, KURIE J M. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer [J]. Cancer Res, 2008, 68(4): 1119-1127.
[5] LIM W T, ZHANG W H, MILLER C R, WATTERS J W, GAO F, VISWANATHAN A, GOVINDAN R, MCLEOD H L. PTEN and phosphorylated AKT expression and prognosis in early- and late-stage non-small cell lung cancer [J]. Oncol Rep, 2007, 17(4): 853-857.
[6] HOSOYA Y, GEMMA A, SEIKE M, KURIMOTO F, UEMATSU K, HIBINO S, YOSHIMURA A, SHIBUYA M, KUDOH S. Alteration of the PTEN/MMAC1 gene locus in primary lung cancer with distant metastasis [J]. Lung Cancer, 1999, 25(2): 87-93.
[7] INAMURA K, TOGASHI Y, NOMURA K, NINOMIYA H, HIRAMATSU M, OKUI M, SATOH Y, OKUMURA S, NAKAGAWA K, TSUCHIYA E, ISHIKAWA Y. Up-regulation of PTEN at the transcriptional level is an adverse prognostic factor in female lung adenocarcinomas [J]. Lung Cancer, 2007, 57(2): 201-206.
[8] PERRONE F, LAMPIS A, ORSENIGO M, DI BARTOLOMEO M, GEVORGYAN A, LOSA M, FRATTINI M, RIVA C, ANDREOLA S, BAJETTA E, BERTARIO L, LEO E, PIEROTTI M A, PILOTTI S. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients [J]. Ann Oncol, 2009, 20(1): 84-90.
[9] TANAKA M, KOUL D, DAVIES M A, LIEBERT M, STECK P A, GROSSMAN H B. MMAC1/PTEN inhibits cell growth and induces chemosensitivity to doxorubicin in human bladder cancer cells [J]. Oncogene, 2000, 19(47): 5406-5412.
[10] ZHOU M, GU L, FINDLEY H W, JIANG R, WOODS W G. PTEN reverses MDM2-mediated chemotherapy resistance by interacting with p53 in acute lymphoblastic leukemia cells [J]. Cancer Res, 2003, 63(19): 6357-6362.
[11] WENG L, BROWN J, ENG C. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways [J]. Hum Mol Genet, 2001, 10(3): 237-42.
[12] XIN J, YANG-DE Z, JI-WEI W, LI-HUA Z, PU Y, XU Z, YU H, LI Y. Preparation and characterization of fluorimetric folate-coupled chitosan nano-carrier [J]. Journal of Central South University: Science and Technology, 2010, 41(1): 161-165. (in Chinese)
[13] DATILES M J, JOHNSON E A, MCCARTY R E. Inhibition of the ATPase activity of the catalytic portion of ATP synthases by cationic amphiphiles [J]. Biochim Biophys Acta, 2008, 1777(4): 362-368.
[14] de MARTIMPREY H, VAUTHIER C, MALVY C, COUVREUR P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: Oligonucleotides and siRNA [J]. Eur J Pharm Biopharm, 2009, 71(3): 490-504.
[15] WU H, CAO Y, WENG D, XING H, SONG X, ZHOU J, XU G, LU Y, WANG S, MA D. Effect of tumor suppressor gene PTEN on the resistance to cisplatin in human ovarian cancer cell lines and related mechanisms [J]. Cancer Lett, 2008, 271(2): 260-271.
[16] STEELMAN L S, NAVOLANIC P M, SOKOLOSKY M L, TAYLOR J R, LEHMANN B D, CHAPPELL W H, ABRAMS S L, WONG E W, STADELMAN K M, TERRIAN D M, LESLIE N R, MARTELLI A M, STIVALA F, LIBRA M, FRANKLIN R A, MCCUBREY J A. Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors [J]. Oncogene, 2008, 27(29): 4086-4095.
[17] WU H, WANG S, WENG D, XING H, SONG X, ZHU T, XIA X, WENG Y, XU G, MENG L, ZHOU J, MA D. Reversal of the malignant phenotype of ovarian cancer A2780 cells through transfection with wild-type PTEN gene [J]. Cancer Lett, 2008, 271(2): 205-214.
[18] WANG S, CHENG Z, YANG X, DENG K, CAO Y, CHEN H, PAN L. Effect of wild type PTEN gene on proliferation and invasion of multiple myeloma [J]. Int J Hematol, 2010, 92(1): 83-94.
[19] LI D M, SUN H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells [J]. Proc Natl Acad Sci USA, 1998, 95(26): 15406-1511.
[20] WENG L P, SMITH W M, DAHIA P L, ZIEBOLD U, GIL E, LEES J A, ENG C. PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death [J]. Cancer Res, 1999, 59(22): 5808-5814.
[21] BRUNI P, BOCCIA A, BALDASSARRE G, TRAPASSO F, SANTORO M, CHIAPPETTA G, FUSCO A, VIGLIETTO G. PTEN expression is reduced in a subset of sporadic thyroid carcinomas: Evidence that PTEN-growth suppressing activity in thyroid cancer cells mediated by p27kip1 [J]. Oncogene, 2000. 19(28): 3146-3155.
[22] KNOBLOCH J, SCHMITZ I, GOTZ K, SCHULZE-OSTHOFF K, RUTHER U. Thalidomide induces limb anomalies by PTEN stabilization, Akt suppression, and stimulation of caspase-dependent cell death [J]. Mol Cell Biol, 2008, 28(2): 529-538.
[23] THOMAS M, KLIBANOV A M. Non-viral gene therapy: Polycation-mediated DNA delivery [J]. Appl Microbiol Biotechnol, 2003, 62(1): 27-34.
[24] SORSCHER S M. Metastatic acinar cell carcinoma of the pancreas responding to gemcitabine, 5-fluorouracil and leucovorin therapy: A case report [J]. Eur J Cancer Care (Engl), 2009, 18(3): 318-319.
[25] KROTZ F, de WIT C, SOHN H Y, ZAHLER S, GLOE T, POHL U, PLANK C. Magnetofection-A highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo [J]. Mol Ther, 2003, 7(5): 700-710.
(Edited by HE Yun-bin)
Foundation item: Project(07JJ3055) supported by the Natural Science Foundation of Hunan Province, China
Received date: 2011-01-18; Accepted date: 2011-05-11
Corresponding author: CHEN Qiong, Professor, PhD; Tel: +86-731-89753056; E-mail: qiongch@yahoo.com.cn
Abstract: To evaluate the feasibility of using magnetic iron oxide nanoparticle as wild PTEN gene carrier for transfection in vitro to reverse cisplatin-resistance of A549/CDDP cells, A549/CDDP cells were transfected with the wild PTEN gene expression plasmid (pGFP-PTEN) by magnetic iron nanoparticle and lipo2000. The transfection efficiency was detected by fluorescence microscope and flow cytometer. The expression levels of PTEN mRNA and protein were detected by reverse transcription polymerase chain reaction (RT-PCR) and immunocytochemistry analysis. The effect of PTEN transfection on cell cycle enhances the sensitivity of A549/CDDP to cisplatin and nanoparticle-mediated transfection has a higher efficiency than that of the liposome-mediated group. The apoptosis level was up-regulated in PTEN transfection group. The magnetic iron oxide nanoparticle could be used as one of the ideal gene carriers for PTEN gene delivery in vitro. PTEN can be an effective target for reversing cisplatin-resistance in lung cancer.


