- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 3.3 Fluorescent properties of complexes
- 3.4 Application in light conversion plastics
- 4 Conclusions▲
- References
- Figure
- Fig.1 Chemical structures of complexes
- Fig.2 SEM images of complexes
- Fig.3 DTA and TG curves of complexes
- Fig. 4 Excitation spectra of complexes
- Fig.5 Emission spectra of complexes
- Fig.6 Relationship between luminescence intensity of film and mass fraction of Eu(Ⅲ) complexes(w)
- Fig.7 Relationship between luminescence intensity and ultraviolet irradiation time with different Eu(Ⅲ) complexes
J. Cent. South Univ. Technol. (2007)01-0062-06
DOI: 10.1007/s11771-007-0013-4
![]()
Syntheses and applications of Eu(III) complexes of 2-thienyltrifluoroacetonate, terephthalic acid and phenanthroline as light conversion agents
ZHAO Xue-hui(赵学辉)1,2, HUANG Ke-long(黄可龙)1, JIAO Fei-peng(焦飞鹏)1,
YANG You-ping(杨幼平)1, LI Zhao-jian(李朝建)1, LIU Zhi-guo(刘志国)2, HU Shun-qin(胡舜钦)2
(1. School of Chemistry and Chemical Engineering, Central South University,Changsha 410083, China;
2. Key Laboratory of Green Packaging and Application Biological Nanotechnology of Hunan Province,Hunan University of Technology, Zhuzhou 412008, China)
Abstract:
A series of europium(Ⅲ) complexes of 2-thienyltrifluoroacetonate (HTTA), terephthalic acid (TPA) and phenanthroline (Phen) were synthesized. The new complexes Eu(TPA)(TTA)Phen and Eu2(TPA)(TTA)4Phen2 were characterized by elemental analysis, IR spectrum, scanning electron microscope and thermal stability analysis. The results show that the thermal stability of the Eu(Ⅲ) complexes increases in the following order: the mononuclear complex Eu(TTA)3Phen, the binuclear complex Eu2(TPA)(TTA)4Phen2, the chain polynuclear complex Eu(TPA)(TTA)Phen. And the formation of the binuclear/polynuclear structure of the new complexes appears to be responsible for the enhancement of their thermal and optical stability. In addition, The fluorescence excitation spectra of these new complexes show more broad excitation bands than that of the complex Eu(TTA)3Phen corresponding to their formation. The enhancement of Eu3+ fluorescence in the new complexes can be observed by the addition of Gd3+. The bright red luminescent plastics can be obtained when the complex EuGd(TPA)(TTA)4Phen2 is added above 0.5% (mass fraction).
Key words:
fluorescent properties; europium; terephthalic acid; 2-thienyltrifluoroacetonate;
1 Introduction
The design of complexes of trivalent rare earth ions is an important subject in the field of supramolecular and coordination polymers, because of the possibility to obtain the complexes having good luminescent properties. An important aspect of this research field is how to optimize the luminescent properties and thermal, optical stability of complexes by a suitable selection of ligands. Europium(Ⅲ) complexes with β-diketones ligands have good luminescent properties[1-2], which have been found important application in light conversion events. As light conversion materials, ultraviolet light is firstly absorbed by β-diketones ligands, then the absorbed energy is transferred to Eu3+ ions and make them send out their characteristic light. β-diketones ligands have a broad absorption band in the region of near ultra-violet, and the energy level of the triplet state matches well with the emission energy level of rare earth europium(Ⅲ), so the energy transfer of β-diketones ligands to europium(Ⅲ) ions is very efficient. These result in the great increase in luminescent intensity of europium(Ⅲ) ion[3-6]. However, the europium(Ⅲ) complexes with β-diketones ligands for practical application in light conversion events are very limited due to their poor thermal, optical stability and low mechanical resistances. In recent years, although both the solubility and fluorescent intensity of the Eu(Ⅲ)- carboxylates were weaker than those of Eu(Ⅲ)-β- diketones, they were used as light conversion agents still, due to their good stability[7-8]. The reason why Eu(Ⅲ)- carboxylates have a good stability is that they can form binuclear complex or one-, two- and three-dimensional coordination polymers[9].
FU et al[10-13] studied the syntheses and luminescent properties of europium(Ⅲ) mononuclear complexes with β-diketones ligands (e.g. 2-thienyltrifluoro acetonate, dibenzoylmethide) and phenanthroline(Phen) or trioctylphosphine oxide. However, the studies on syntheses and luminescent properties of europium(Ⅲ) complexes of 2-thienyltrifluoroacetonate with terephtha- lic acid (TPA) and phenanthroline have not been reported yet.
The aim of this study is to study the synthesis and fluorescence properties of the new binuclear complex Eu2(TPA)(TTA)4(Phen)2 and chain polynuclear complex Eu(TPA)(TTA)Phen, using bridging ligand TPA to link europium(III) ions to form binuclear or polynuclear. And their thermal and optical stability were investigated. In addition, IR spectrum, scanning electron microscope and fluorescence spectra of these complexes were also studied.
2 Experimental
2.1 Reagents and apparatus
99.99% Eu2O3 and 99.98% Gd2O3 were purchased from Jiangxi South Rare Earth Metals Institute in China. 2-thienyltrifluoroacetonate (HTTA), terephthalic acid (TPA), phenanthroline (Phen) and other reagents were all analytical grade and used without further purification.
The elemental analyses of C, H and N were performed on an American Perkin-Elmer 2400 Ⅱ CHNSLO elemental analyzer. The mass fraction of Eu(Ⅲ) was determined by complexometric titration with EDTA. The infrared (IR) spectra were measured on a Nicolet-550 spectrophotometer (American Perkin- Elmer) using KBr pellets in the range of 4 000-400 cm-1 at room temperature. The scanning electronic microscope (SEM) was obtained on a microscope JSM-5600LV along with the gold sputtering technique. The differential thermoanalysis-thermogravimetric (DTA-TG) were performed on a SHDT-40 thermoanalyticmeter using aluminum crucibles with about 18.4 mg and 18.0 mg samples respectively, under dynamic synthetic air atmosphere (40 mL/min) and heating rate of 10 ℃/min at 30-600 ℃. An SPEX FL-2T2 spectrofluorometer was used to record the fluorescence excitation and emission spectra of the complexes using a 450 W Xenon lamp as excitation source. This apparatus was controlled by a DM3000F spectroscopic computer.
2.2 Synthesis of complexes
The complex Eu2(TPA)(TTA)4Phen2 was prepared in the following steps. In the first place, standard solution (0.1 mol/L) of Eu3+ (or Gd3+) was prepared by dissolving Eu2O3 (or Gd2O3) in hot hydrochloric acid, evaporating up to syrup and diluting with ethanol to a desired volume. HTTA, TPA and Phen were dissolved in ethanol with molar ratio of 4?1?2. Subsequently EuCl3 and HTTA solutions were mixed with molar ratio of 1?2, adjusting pH value to 5.0, stirred and refluxed for 40 min keeping temperature in water-bath. Then according to molar composition of formula Eu2(TPA)(TTA)4Phen2, Phen and TPA solutions were added dropwise, keeping pH value 6.5, stirred and refluxed until the appearance of an orange precipitate. The solid product was filtered, washed and recrystallized in ethanol.
The Eu2(TPA)3Phen2 and Eu(TPA)(TTA)Phen complexes were prepared by the similar process as Eu2(TPA)(TTA)4Phen2, except that the products were a pale yellow precipitate.
The complexes Eu2(1-x)Gd2x(TPA)(TTA)4Phen2 and Eu1-xGdx(TPA)(TTA)Phen were prepared by the similar process as Eu2(TPA)(TTA)4Phen2, except that the mixture solution of EuCl3 and GdCl3 was used instead of the EuCl3. The products obtained were an orange or a pale yellow precipitate.
The complex Eu(TTA)3Phen was synthesized by the similar process as described in Ref[14]. The product obtained was an orange precipitate.
3 Results and discussion
3. 1 Composition of complexes
The mass fraction of Eu(Ⅲ) was determined by complexometric titration with EDTA. The elemental analysis data of C, H, N and Eu(Ⅲ) for complexes are listed in Table 1, which are similar to the calculated values.
3. 2 Characterization of complexes
Table 2 shows IR spectra data of complexes. The presence of carboxylate groups is definitely confirmed by both asymmetric stretching bands at 1 552-1 569 cm-1 and symmetric stretching bands at 1 397- 1 405 cm-1 respectively in Eu(III) complexes. The separations (Δυ=υas-υs) between υas (COO) peaks and υs(COO) peaks are in the range of 155-164 cm-1 in Eu(Ⅲ) complexes, which is attributed to the bidentate chelating, bidentate bridging or tridentate chelating-bridging coordination modes of carboxylate groups with Eu3+, since the separations (Δυ=υas-υs) in
Table 1 Elemental analysis data of complexes
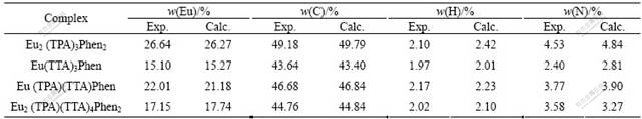
Table 2 IR spectra data of complexes
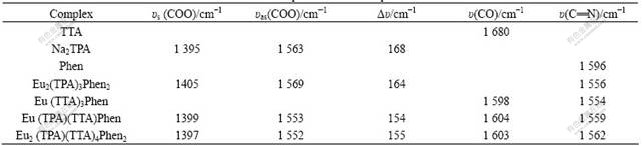
Eu(Ⅲ) complexes are lower than that in Na2TPA (Δυ= 168 cm-1). Furthermore, owing to the great steric hindrance of the complexes Eu(TPA)(TTA)Phen and Eu2(TPA)(TTA)4Phen2, both bidentate bridging coordination and tridentate chelating-bridging coordination of carboxylate groups with rare earth ions become more difficult than the bidentate chelating coordination does. Thus, the coordination mode of carboxylate groups with rare earth ions is mainly the bidentate chelating coordination mode in the complexes Eu(TPA)(TTA)Phen and Eu2(TPA)(TTA)4Phen2, and their chemical structures proposed are shown in Fig.1. In addition, the IR spectra also show a displacement of υ(C==O) stretching from about 1 680 cm-1, in free TTA ligand, to approximately 1 603 cm-1 in the complexes, and a displacement of υ(C==N) stretching from 1 596 cm-1, in free Phen ligand, to approximately 1 559 cm-1 in the complexes, indicating that Eu(Ⅲ) ion is coordinated by the oxygen or nitrogen atoms[15].
The SEM images of complexes Eu(TPA)(TTA)Phen and Eu2(TPA)(TTA)4Phen2 are shown in Fig.2. For the
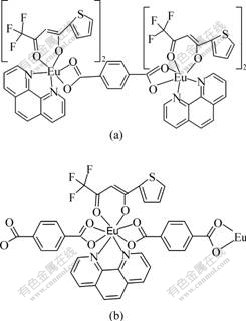
Fig.1 Chemical structures of complexes
(a) Eu2(TPA)(TTA)4 Phen2; (b) Eu(TPA)(TTA)Phen
binuclear complex Eu2(TPA)(TTA)4Phen2, the formation of the agglomerated structure can be obtained by intermolecular force, one of them behaving like a supermolecule recovering the other, and for the polynuclear complex Eu(TPA)(TTA)Phen, the needle/ stripe shaped structure can be obtained by chain conglomeration.

Fig.2 SEM images of complexes
(a) Eu2(TPA)(TTA)4Phen2; (b) Eu(TPA)(TTA)Phen
The DTA and TG curves of Eu(TPA)(TTA)Phen and Eu2(TPA)(TTA)4Phen2 are shown in Fig.3. From DTA curves it can be seen that Eu2(TPA)(TTA)4Phen2 (Fig.3(a)) complex melts at about 210 ℃ and Eu(TPA)(TTA)Phen (Fig.3(b)) complex melts at about 229 ℃, both with no decomposition before the melting point. The thermal decomposition temperature of Eu(TPA)(TTA)Phen is higher than that of Eu2(TPA)- (TTA)4Phen2. In addition, the temperature of the thermal decomposition of the mononuclear complex Eu(TTA)3Phen[14] is at 260-360 ℃. So the thermal stability for Eu(Ⅲ) complexes decreases in the following order: the chain polynuclear complex Eu(TPA)(TTA)Phen>the binuclear complex Eu2(TPA)(TTA)4Phen2>the mononuclear complex Eu(TTA)3Phen. The formation of the binuclear/ polynuclear structure of the complexes appears to be responsible for the enhancement of the thermal stability of the complexes. Moreover, the TG curves do not present a little water loss at 50-200 ℃, indicating that two new complexes are in anhydrous form. This is corroborated by IR spectra and elemental analysis results.
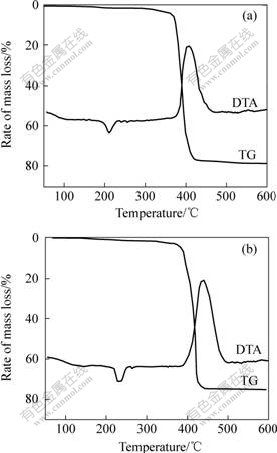
Fig.3 DTA and TG curves of complexes
(a) Eu2(TPA)(TTA)4Phen2; (b) Eu(TPA)(TTA)Phen
3.3 Fluorescent properties of complexes
The excitation spectra of Eu(Ⅲ) complexes, recorded in the spectral range of 220-450 nm by monitoring the emission at the hypersensitive 5D0→7F2 transition, are shown in Fig. 4. The excitation spectrum of Eu2(TPA)3 Phen2 consists of a broad band ranging from 230 to 350 nm with the maximum excitation wavelengths at 328 nm, corresponding to the absorber of ligand TPA. The excitation spectrum of Eu(TTA)3Phen shows a strong broad band ranging from 275 to 425 nm with the maximum excitation wavelengths at 388 nm. However, the data of Eu(TPA)(TTA)Phen and Eu2(TPA)(TTA)4Phen2 complexes exhibit more broad bands with maximum excitation peaks respectively at 376 nm for Eu(TPA)(TTA)Phen and at 382 nm for Eu2(TPA)(TTA)4Phen2. In addition, in contrast to Eu(TTA)3Phen, the excitation band of the TTA in Eu2(TPA)(TTA)4Phen2 shows a peak position blue shift, and it is same case for Eu(TPA)(TTA)Phen. Furthermore, compared with Eu2(TPA)(TTA)4Phen2, the excitation band of Eu(TPA)(TTA)Phen also shows that a peak position blue shift occurs. These facts show the presence of the different orientation and coordination environ- ments for TPA and TTA ligands in different complexes, corresponding to the formation of Eu(Ⅲ) complexes.
The emission spectra of Eu(Ⅲ) complexes recorded in the range of 560-710 nm with the maximum excitation wavelengths at room temperature, are shown in Fig.5. It can be seen from Fig.5 that five typical Eu(Ⅲ) emission bands appear at about 582, 593, 615, 654 and 704 nm, corresponding to 5D0→7F0, 5D0→7F1, 5D0→7F2, 5D0→7F3 and 5D0→7F4, respectively. Of all the fluorescent emissions for Eu(III) complexes, the relative fluorescence intensity of 5D0→7F2 is the strongest. In addition, it can be also seen from Fig.5 that the ratios of the relative intensity 5D0→7F2 and 5D0→7F1 in the complexes Eu(TPA)(TTA)Phen and Eu2(TPA)(TTA)4- Phen2 are higher than that of Eu2(TPA)3Phen2. And the symmetry of the new complexes changes much less, i.e. the forced electric dipole transition (5D0→7F2) of Eu3+ in the new complexes is strengthened, and the color purity of the assembly luminescence is significantly improved.
Table 3 shows the relative fluorescence intensity for the interesting Eu(III) complexes studied, and their fluorescence intensities (5D0→7F2) decrease in the following order: EuGd(TPA)(TTA)4Phen2>Eu(TTA)3- Phen>Eu0.5Gd0.5(TPA)(TTA)Phen>Eu2(TPA)(TTA)4- Phen2>Eu(TPA)(TTA)Phen>Eu(TPA)3Phen. The results demonstrate that the fluorescence intensity of Eu(Ⅲ) complexes is influenced by the structure of complexes and the coordination environments of ligands. The fluorescence enhancement of EuGd(TPA)(TTA)4- Phen2 complex may be concerned with the formation of the binuclear structure.
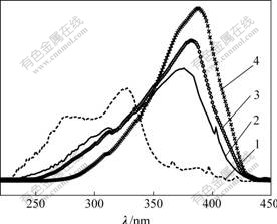
Fig. 4 Excitation spectra of complexes
1—Eu2(TPA)3Phen2; 2—Eu(TPA)(TTA)Phen;3—Eu2(TPA)(TTA)4Phen2; 4—Eu(TTA)3Phen
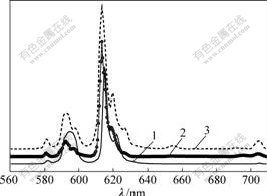
Fig.5 Emission spectra of complexes
1—Eu2(TPA)3Phen2; 2—Eu(TPA)(TTA)Phen;3—Eu2(TPA)(TTA)4Phen2
3.4 Application in light conversion plastics
The complexes EuGd(TPA)(TTA)4Phen2 and Eu0.5Gd0.5(TPA)(TTA)Phen, which have strong emission intensity and good thermal stability, were used as an additive for high pressure polyethylene, and red fluorescence plastics under ultraviolet excitation was obtained. Using the fluorescence plastics, light conversion agriculture film can be prepared. This film can converse ultraviolet light to red light, which can accelerate the process of photosynthesis and metastasis of plants, and improve the out-put and quality of agricultural products. In order to investigate the lumines-cent properties of the film, the relationship between the content of Eu(III) complexes in film and the fluorescence intensity of the film (0.1 mm in thickness) using 365 nm ultraviolet light are shown in Fig.6. It can be seen that the fluorescence intensity of the film increases with the increase of the content of Eu(III) complexes. When the content of Eu(III) complexes is above 0.5%(mass fraction), a bright red fluorescence plastic film can be obtained.
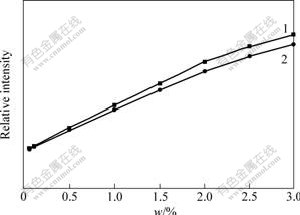
Fig.6 Relationship between luminescence intensity of film and mass fraction of Eu(Ⅲ) complexes(w)
1—EuGd(TPA)(TTA)4Phen2;2—Eu0.5Gd0.5(TPA)(TTA)Phen
In order to investigate the optical stability of Eu(III) complexes, some optical stability tests were carried out using 360 nm ultraviolet light. The results are shown in Fig.7. It can be seen that the optical stability of the Eu(III) complexes decreases in the following order: the polynuclear complex Eu0.5Gd0.5(TPA)(TTA)Phen>the binuclear complex EuGd(TPA)(TTA)4Phen2>the mononuclear complex Eu(TTA)3Phen, and the optical stability of Eu(III) complexes in film is higher than that of the corresponding pure Eu(III) complexes.
Table 3 Fluorescence spectra data of complexes
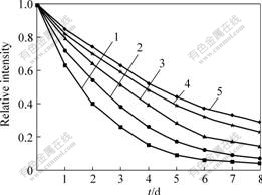
Fig.7 Relationship between luminescence intensity and ultraviolet irradiation time with different Eu(Ⅲ) complexes
1—Eu(TTA)3Phen; 2—EuGd(TPA)(TTA)4Phen2;3—Eu0.5Gd0.5(TPA)(TTA)Phen;4—Film of EuGd (TPA)(TTA)4Phen2;5—Film of Eu0.5Gd0.5 (TPA)(TTA)Phen.
4 Conclusions
1) A series of new light conversion complexes Eu2(TPA)(TTA)4Phen2, Eu2(1-x)Gd2x(TPA)(TTA)4Phen2 and Eu(TPA)(TTA)Phen and Eu1-xGdx(TPA)(TTA)Phen, showing strong red fluorescence and the good thermal and optical stability, are synthesized.
2) The thermal and optical stability for Eu(III) complexes decreases in the following order: Eu(TPA)(TTA)Phen>Eu2(TPA)(TTA)4Phen2>Eu- (TTA)3Phen. The formation of the binuclear/ polynuclear structure of the complexes appears to be responsible for the enhancement of the thermal and optical stability of the complexes.
3) The relative fluorescence intensity for Eu(III) complexes decreases in the following order: EuGd(TPA)(TTA)4Phen2>Eu(TTA)3Phen>Eu0.5Gd0.5- (TPA)(TTA)Phen>Eu2(TPA)(TTA)4Phen2>Eu(TPA)- (TTA)Phen>Eu(TPA)3Phen
4) The light conversion agriculture film with good luminescent properties can be prepared by using the complex EuGd(TPA)(TTA)4Phen2, which has strong fluorescence together with the good thermal and optical stability, as the additive of high pressure polyethylene.
References
[1] LIU Hong-guo, SEONGTAE P, KIWAN J, et al. Influence of ligands on the photoluminescent properties of Eu3+ in europium β-diketonate/ PMMA-doped systems [J]. Journal of Luminescence, 2004, 106: 47-55.
[2] YANG J H, ZHU G Y, WU B. Enhanced luminescence of the europium/terbium/thenoyltrifluoroacetone/1,10-phenanthroline/surfactant system and its analytical application[J]. Anal Chim Acta, 1987, 198: 287-291.
[3] BRITO H F, MALTA O L, MENEZES J F S. Luminescent properties of diketonates of trivalent europium with dimethylsulfoxide[J]. Jourmal of Alloys and Compounds, 2000, 300: 336-339.
[4] YANG J H, ZHOU H B, REN X Z, et al. Fluorescence enhancement of the Eu-Tb-Benzoylacetone-phenanthroline system[J]. Anal Chim Acta, 1990, 238: 307-402.
[5] YANG J H, REN X Z, ZHOU H B, et al. Enhanced luminescence of the europium(III)-dibenzoylmethane-ammonia-acetone system and its analytical application to the determination of europium ion[J]. Analyst, 1990, 115: 1505-1509.
[6] YANG J H, ZHU G Y, WU H. Application of the co-luminescence effect of rare earth: simultaneous determination of trace amounts of samarium and europium in solution[J]. Analyst, 1989, 114: 1417-1421.
[7] LI B, MA D , ZHANG H J, et al. Electroluminescence devices based on monohexadecyl phthalate terbium [J]. Thin Solid Films, 1998, 325: 259-263.
[8] WANG Ze-min. Advance in study of light transform farm film doped with rare earth in China[J]. Chinese Rare Earths, 2000, 21(5): 55-59.(in Chinese)
[9] WAN Y H, ZHANG L P, JIN P L. Three new lanthanide coordination polymers containing isophthalate and 1, 10-phenanthroline[J]. Journal of Molecular Structure, 2003, 658: 253-259.
[10] FU Y J, WONG T K S, YAN Y K, et al. Syntheses, structures and Luminescent properties of Sm(III) and Eu(III) chelates for organic electroluminescent device applications[J]. Journal of Alloys and Compounds, 2003, 358: 235-244.
[11] FU Y J, WONG T K S, YAN Y K, et al. Syntheses, characterization and luminescent properties of Eu(III) complex[J]. Thin Solid Films, 2002, 417: 78-84.
[12] ZHANG Ren-jie, LIU Hong-guo, ZHANG Cheng-ru, et al. Influence of several compounds on the fluorescence of rare earth complexes Eu(TTA)3Phen and Sm(TTA)3Phen in LB films[J]. Thin Solid Films, 1997, 302: 223-230.
[13] WANG Zheng-xiang, SHU Wan-yin, ZHOU Zhong-cheng, et al. Fluorescence properties and application of doping complexes Eu1-xLx(TTA)3Phen as light conversion agents[J]. J Cent South Univ Technol, 2003, 10 (4): 342-346.
[14] JI Xiang-ling, LI Bin, JIANG Shi-chun, et al. Luminescent properties of organic- inorganic hybrid monoliths containing rare-earth complexes[J]. Journal of Non-crystalline Solids, 2000, 275: 52-58.
[15] CLAUDIA F C F M, CLAUDIA S T, HERMI F B, et al. Synthesis and luminescent properties of supermolecules of β–diketonate of Eu(III) and crown ethers as ligands[J]. Journal of Solid State Chemistry, 2003, 171: 189-194.
Foundation item: Project(20576142) supported by the National Natural Science Foundation of China; Project (05B075) supported by the Foundation of Hunan Provincial Education Department for Young Scholars
Received date: 2006-05-28; Accepted date: 2006-07-29
Corresponding author: HUANG Ke-long, Professor; Tel: +86-731-8879850; E-mail: klhuang@mail.csu.edu.cn
- Syntheses and applications of Eu(III) complexes of 2-thienyltrifluoroacetonate,terephthalic acid and phenanthroline as light conversion agents


