
Hot deformation behavior of rare earth magnesium alloy without pre-homogenization treatment
MA Ming-long(马鸣龙), ZHANG Kui(张 奎), LI Xing-gang(李兴刚),
LI Yong-jun(李永军), ZHANG Kang(张 康)
State Key Laboratory for Fabrication and Processing of Nonferrous Metals,
Beijing General Research Institute for Nonferrous Metals, Beijing 100088, China
Received 12 June 2008; accepted 5 September 2008
Abstract:
The behavior and structure evolvement of as-cast Mg-Gd-Y-Nd-Zr magnesium alloy during the hot deformation process were discussed. The flow stress behavior of magnesium alloy over the strain rate range of 0.002-1 s-1 and the temperature range of 573-723 K was researched on Gleeble-1500D hot simulator under the maximum deformation degree of 60%. The experimental results show that the relationship between stress and strain is obviously affected by the strain rate and deformation temperature. The important softening mechanisms are eutectic melting and discontinuous dynamic recrystallization (DDRX) during deformation. The fragments of eutectic melting along the boundaries can turn round so as to take effect of the slippage between grains. The flow stress of Mg-7Gd-5Y-1.2Nd-Zr magnesium alloy during high temperature deformation can be represented by a Zener-Hollomon parameter in the hyperbolic Arrhenius-type equation. The strain coefficient n and deformation activation energy Q are evaluated by linear regression analysis. A, α and n in the analytical expressions of σ are fitted to be 2.401 93×1015, 0.017 3 MPa-1 and 3.218 19, respectively. The hot deformation activation energy of alloy during hot deformation is 234.950 58 kJ/mol. The results also show that the structure of primitive microstructure has an effect on the plastic deformation.
Key words:
rare-earth magnesium alloy; hot-deformation; microstructure; flow stress; constitutive equation;
1 Introduction
Because of low density, high specific strength and high specific rigidity, magnesium and magnesium alloys have been paid much attention in aerospace and automobile industry in recent years[1]. However, due to the hexagonal close-packed structure and low stacking fault energy[2-3], magnesium alloys have poor plastic deformation property. This is why the cast magnesium alloys are developed prior to wrought magnesium alloys. It has been reported that rare earth elements could remarkably improve the mechanical ability of magnesium at room and high temperatures[4]. So, magnesium alloys with rare earth elements attract much interest. Homogenization treatment is very important to the alloy before plastic deformation because it can reduce segregation forming in solidification, and homogenized ingots can be extruded easier and faster and have better surface finish and higher tensile properties than as-cast billets[5-6]. Some researchers focused on the relationship between the homogenization and the microstructures before the deformation, and many cared about the effect of homogenization on the recrystallization resistance of the investigated alloys after the deformation processing[7]. However, alloys transformed directly without homogenization were less studied[7-8]. After deformation without homogenization, the eutectic structure dispersoids distribute around the grains, the specific surface area is larger than before and elements diffuse quickly, so it might need less time at high temperature for homogenization.
In this work, the plastic deformation behaviors of a Mg-7Gd-5Y-1.2Nd-1Zr alloy were presented. The alloy deformed directly without any treatment and this might be different from other studies. The microstructure evolution was studied, which provided both a theoretical and an experimental background for the calculation of mechanical parameters and technique processing optimization of magnesium alloy in plastic deformation.
2 Experimental
The basic alloy was fabricated in a medium- frequency induction furnace under RJ-2 flux-refining. The high purity elements Gd, Y, Nd and Zr in the form of Mg-30%Zr master alloy were added to melt and then cast into a steel mould pre-heated to 300 ℃. Cylindrical specimens for the compression tests were machined to 10 mm in diameter and 15 mm in height utilizing electro- discharge machine without pre-homogenization. In order to decrease the friction and maintain uniform deformation, thin graphite sheets were placed between the compression specimen and the die during the test. Isothermal hot compression tests were performed on a Gleeble-1500D thermal mechanical simulator in the temperature range of 573-723 K and at constant strain rates of 0.002, 0.01, 0.1 and 1 s-1, respectively. The specimens were heated to the deformation temperature at 5 ℃/s, after heat preservation for 2 min. The maximum comparative gauge reductions of all the specimens were 60%. As soon as the deformation was completed, the specimen was frozen by cool water in order to keep the microstructure at high temperature. And then, the specimens were cut symmetrically into two parts parallel to the compression axis. Center parts of the specimens where the specimens had the largest deformation were chosen as the investigation area of interest. Also, the regions vertical to the compression axis were the objects to be studied. After adequate grinding and polishing, specimens for optical observation were etched using solution of 4% HNO3 with ethanol. Microstructures of the specimens were analyzed with Carl Zeiss Axiovet 2000MAT optical microscope and Hitachi X650 scanning microscope attached with energy dispersive spectrometer. Phase constitutions of the alloy were analyzed by PANalytical X’Pert PRO MPD X-ray powder diffractometer.
3 Results and discussion
3.1 Microstructure of as-cast alloy
The typical microstructures and XRD pattern of as-cast alloy are shown in Fig.1. It could be seen that alloy structure was composed of primarily α-Mg matrix and eutectic β phase. The XRD pattern and SEM with EDS (Fig.2) of the alloy indicated that β phase was organized by Mg24Y5, Mg5.05Gd, Mg41Nd5 and Mg3Gd.
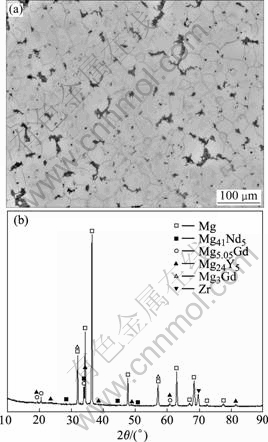
Fig.1 Microstructure and X-ray diffraction patter of as-cast alloy
The β phase formed by Mg-Gd-Y-Nd four elements precipitated as discontinuous network primarily at grain boundaries. Grain refining by the addition of Zr was extremely efficient[9], which in fact was the most efficient grain refining additive ever reported for metallic alloys. SEM image showed that Zr was in the form of elementary substance.
3.2 True stress—true strain curves
The typical true stress—true strain curves of as-cast magnesium alloy at different deformation temperatures and various strain rates are present in Fig.3. Flow stress behavior of alloy is sensitively dependent of the strain rate and temperature. In general, the flow stress diminishes with rising temperature and decreasing strain rate. At a low temperature of 573-623 K, the flow stress increased at the high strain rate obviously and the alloy broke at 0.1 s-1. Because of its HCP structure, magnesium alloy has limited ductility and the work hardening plays a main role on the plastic deformation of alloy. When the strain rate was 1 s-1 at 573 K, the curve was special. With rising the temperature to the intermediate level, the flow stress decreased after critical
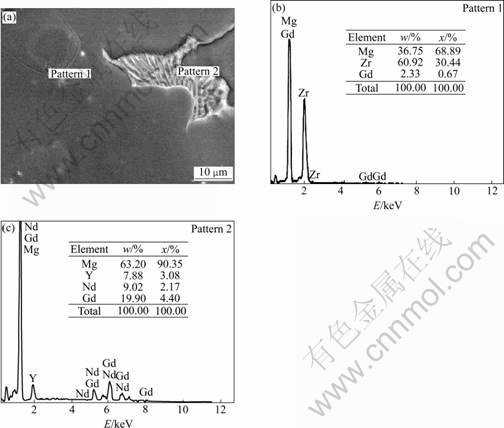
Fig.2 SEM and EDS showing microstructure and constitution of as-cast alloy
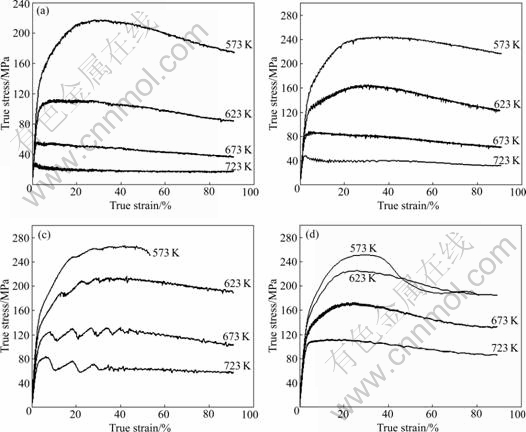
Fig.3 True stress—true strain curves of Mg-Gd-Y-Nd-Zr as-cast alloy at different situation: (a) ![]() 0.002 s-1; (b)
0.002 s-1; (b) ![]() 0.01 s-1; (c)
0.01 s-1; (c) ![]() 0.1 s-1; (d)
0.1 s-1; (d) ![]() 1 s-1
1 s-1
strain. The curves showed the characteristics of dynamic recrystallization which could be divided into two types: continuous dynamic recrystallization (DRX) and discontinuous dynamic recrystallization (DDRX). One of the differences between them was that the continuous DRX had one peak stress, but DDRX had more than one peak stress[10]. At high temperature (above 673 K), the flow stress increased to a maximum value at first and decreased to a stable state finally. At the point of peak stress, the work hardening and softening became balance and then the softening was more pronounced, causing the stress to decrease.
3.3 Model of flow stress
The Arrhenius equation is widely used to describe the relationship among the strain rates, flow stress and temperature, especially at high temperature[11-12]. Hot deformation is a process of thermal activation and lots of investigations have been performed to characterize the material deformation behavior. ZENER and HOLLOMON found that the stress—strain relation of steel depended on deformation temperature and strain rate. They proposed that a material flow stress model could be expressed as a function of strain, strain rate and temperature, and the relationship of temperature and strain rate could be also denoted by a parameter Z [13-14]:
σ=σ(Z, ε) (1)
Z=![]() exp(Q/RT) (2)
exp(Q/RT) (2)
where σ is the flow stress; ![]() is the strain rate; Q is the average apparent activation energy of deformation; R is the ideal gas constant (R=8.314 J/(mol?K)); T is the deformation temperature; and Z is Zener-Hollomon parameter, the physical meaning of which is the so-called temperature compensated strain rate parameter[15].
is the strain rate; Q is the average apparent activation energy of deformation; R is the ideal gas constant (R=8.314 J/(mol?K)); T is the deformation temperature; and Z is Zener-Hollomon parameter, the physical meaning of which is the so-called temperature compensated strain rate parameter[15].
Commonly, the relationship between the strain rate and flow stress conforms to the following equations[14]:
![]() (3)
(3)
![]() (4)
(4)
![]() (5)
(5)
where n1, A1, β, A2, A and α (α=β/n) are material constants. Eqn.(3) and Eqn.(4) are commonly applied to low stress and high stress, respectively. Eqn.(5) in the hyperbolic sine law proposed by SELLARS and TEGART is generally used to describe the flow stress and deformation activation behavior over a wide range of temperature and strain rate, which is a modified Arrhenius relationship that contains the deformation activation energy (Q) and the deformation temperature. Therefore, a constitutive equation for the magnesium alloy was proposed by employing hyperbolic-sine-type equation. A numerical curve-fitting method was used to yield values of the kinetic parameters.
Considering the definition of the hyperbolic law, the flow stress could be written as the function of the Zener-Hollomon parameter from Eqns.(2) and (5):
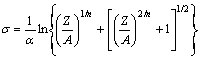 (6)
(6)
By taking natural logarithm, Eqn.(5) could be written as
![]() (7)
(7)
Because the value of α is independent on the temperatures and strain levels[16-17], it could be easily obtained. The hot deformation activation energy Q (J/mol) serving as indicator of deformation difficulty degree in plasticity deformation theory could be calculated by the following equation:
![]() (8)
(8)
By linear regression of the relations of lnσ—![]() (Fig.4(a)) and σ—
(Fig.4(a)) and σ—![]() (Fig.4(b)) at different tempera- tures, α was determined as 0.009 895 609 MPa-1. Fig.4(c) shows the relationship between ln[sinh(ασ)] and
(Fig.4(b)) at different tempera- tures, α was determined as 0.009 895 609 MPa-1. Fig.4(c) shows the relationship between ln[sinh(ασ)] and ![]() and Fig.4(d) shows the relationship between ln[sinh(ασ)] and 1/T. The regression coefficients of several groups of parallel and straight lines are all greater than 0.96. The hot deformation activation energy Q of the alloy is determined through substituting the slopes of Fig.4(a) and Fig.4(b) into Eqn.(8), and the calculated value is obtained to be about 234.950 59 kJ/mol.
and Fig.4(d) shows the relationship between ln[sinh(ασ)] and 1/T. The regression coefficients of several groups of parallel and straight lines are all greater than 0.96. The hot deformation activation energy Q of the alloy is determined through substituting the slopes of Fig.4(a) and Fig.4(b) into Eqn.(8), and the calculated value is obtained to be about 234.950 59 kJ/mol.
By substituting Eqn.(5) into Eqn.(2) and taking the natural logarithm of both sides, the relationship between ln[sinh(ασ)] and lnZ is plotted in Fig.5 through linear regression.
As shown in Fig.5(a), the slope corresponding to the value of stress exponent n could be obtained, and the intercept corresponding to 1nA is 39.161 7. The regression coefficient reached 0.987 58. In order to obtain an optimum value, we substituted n to α=β/n and calculated again. After three times, stress exponent n was obtained to be 3.218 19, and the intercept corresponding to ln A as 35.415 05. The regression coefficient reached 0.989 19 (Fig.5(b)). This meant the results above gave better approximation and the flow stress of the magnesium alloy could be symbolized by a hyperbolic- sine-type equation expressed as follows:
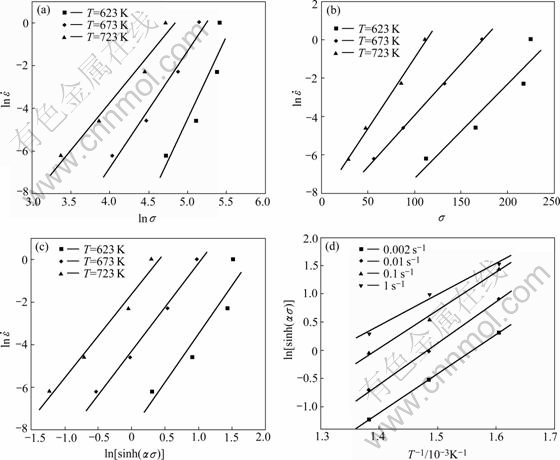
Fig.4 Relationship among peak stress, strain rate and temperature during plastic deformation: (a) lnσ—![]() ; (b) σ—
; (b) σ—![]() ; (c) ln[sinh(ασ)]—
; (c) ln[sinh(ασ)]—![]() ; (d) ln[sinh(ασ)]—T -1
; (d) ln[sinh(ασ)]—T -1
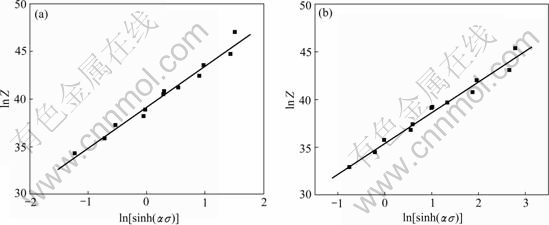
Fig.5 Relationship between α and Zener-Hollomon parameter: (a) For one time; (b) After three times
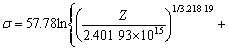
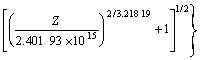 (9)
(9)
![]() 2.401 9×1015[sinh(0.017 3 σ)]3.218 19×
2.401 9×1015[sinh(0.017 3 σ)]3.218 19×
exp(-234.950 59×103/RT) (10)
3.4 Deformed microstructures evolved at same strain
Typical microstructures of the magnesium alloy without pre-homogenization treatment at strains of 60% under 0.1 s-1 at different temperatures are shown in Fig.6.
It was seen that the evolution of microstructure was temperature dependent. At all deformation temperatures, almost all of the grains were obviously elongated in the perpendicular direction to the compression axis. The boundaries of elongated grains were nearly straight and no apparent recrystallization grains were observed. The one thing we could find was that the widths of the grains were different at various temperatures. At 623 K, the specimen had narrow grains. With increasing the temperature, the grains became wider. Deformation was the process of competition between work hardening and dynamic softening that happened and competed with each other at the same time. Dynamic softening might be led by deformation heating and also by microstructure
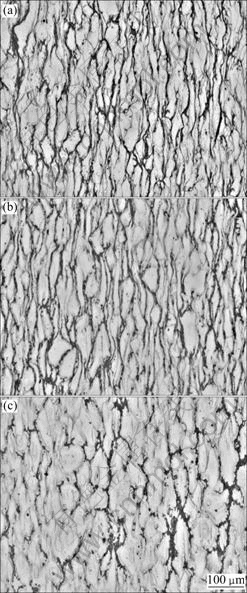
Fig.6 Effect of temperature on microstructure of as-cast magnesium alloy at same strain with strain rates of 0.1 s-1: (a) 623 K; (b) 673 K; (c) 723 K
instabilities, such as dynamic recovery (DRV), dynamic recrystallization, and texture formation[15]. Both DRX and DRV were of importance in determining the hot deformation behavior of alloy to a large extend as two critical softening mechanisms. But in this experiment, the important softening mechanisms were eutectic melting and DDRX during deformation. From the microstructure of the alloy, we found a lot of eutectic fragments along the grain boundaries and a little DDRX in perpendicular direction to compression axis (Fig.7). This was the reason why grains became thick when rising temperature. At low temperature, eutectic structure and grain boundaries coalesced well. So the grains were elongated greatly during deforming. When rising temperature up, the eutectic structure became weak and it could be destroyed easily, leaving eutectic fragments. The fragments along the boundaries could turn round so as to take effect of the slippage between grains and the grain structure looked thicker a little. As for DDRX, the true stress—true strain curves of 0.1 s-1 showed up clearly and the course of DDRX was mainly decided by the strain rates in this experiment. After deformation in the proper scheme, eutectic structure was destroyed, leaving the fragments along the boundary. There might need less time for the elements to diffuse in homogenization because the specific surface area was larger than before.
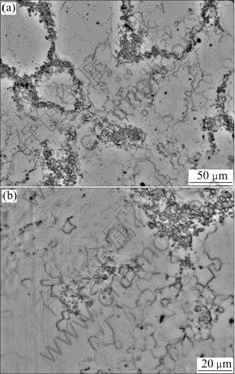
Fig.7 Microstructure of DDRX in perpendicular direction to compression axis at 673 K and 0.002 s-1: (a) In low magnification; (b) In high magnification
Meanwhile, strain rate also had remarkable effect on the microstructure of hot deformation. At high strain rate, two aspects could be brought. On one hand, as dislocations had no enough time to consume or continually generated, the accumulated energy increased, and at last the specimen broke up; on the other hand, the effect of deformation heating on flow stress increased with increasing strain rate, which could soften the matrix. When the strain rate was relatively low, this influence could be neglected and the compression process could be considered as an isothermal process. While the equivalent strain rate was high, this influence was important and the compression process could be approximately considered as an adiabatic process[18]. It could lower down the strain stress during the deformation process since the deformation heating could make the dislocations consumed and soften the eutectic structure. At 573 K, the specimen was broken at strain rate of 0.1 s-1 and the external surface of the specimen at strain rate of 1 s-1 was fine although there was crossing crack inside (Fig.8). We could conclude that the deformation heating might delay the crack development.
Fig.9(a) shows the maximum temperatures measured during hot deformation in different schemes. It could be seen that when the strain rate was large, the effect of deformation heating was more obviously. When the strain rate was 1 s-1, temperature rose up by more than 40 K and the effect could not be neglected. Generally, when the strain rate was relatively low, the possibility of failure was less, but this rule was not much fit for this experiment. The hot deformation activation energy Q could also prove it, as shown in Fig.9(b). The value of Q at 0.1 s-1 was higher than that at 1 s-1. This indicated that at the strain rate of 0.1 s-1, the specimen had more danger and might not be suitable for the magnesium alloy to deform.
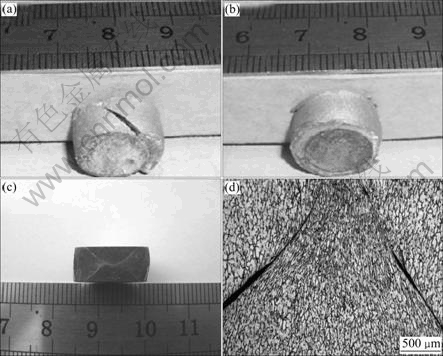
Fig.8 Pictures of samples at different strain rates: (a) 573 K, 0.1 s-1, 60%; (b) 573 K, 1 s-1, 60%; (c) Vertical section macrostructure of sample b; (d) Vertical section microstructure of sample b

Fig.9 Temperature measured at different strain rates (a) and relationship between Q and strain rates at different temperatures (b)
4 Conclusions
1) The GWN751K magnesium alloy was composed of primary α-Mg matrix and eutectic β phase. The XRD pattern and SEM with EDS of the alloy indicated that β phase was organized by Mg24Y5, Mg5.05Gd, Mg41Nd5 and Mg3Gd.
2) The flow stress of GWN751K magnesium alloy could be symbolized by a hyperbolic-sine-type equation expressed as follows:
![]()
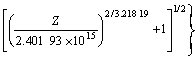
or
![]() 2.401 9×1015[sinh(0.017 3 σ)]3.218 19?
2.401 9×1015[sinh(0.017 3 σ)]3.218 19?
exp(-234.950 59×103/RT)
3) The important softening mechanisms were eutectic melting and DDRX during deformation. The eutectic structure fragments along the boundaries could turn round, which took effect of the slippage between grains.
4) The effect of deformation heating on flow stress increased with increasing strain rate. While the equivalent strain rate was high, this influence could not be neglected.
References
[1] LIU Zheng, ZHANG Kui, ZENG Xiao-qin. Theory basis and application of magnesium matrix light alloy [M]. Beijing: Mechanical Industry Press, 2002: 222. (in Chinese)
[2] WILEY J. Magnesium and its alloys [M]. USA: Sons Inc. 1960.
[3] CAHN R W. Materials science and technology: Structure and properties of nonferrous alloys (Vol.8) [M]. Beijing: Science Press, 1999.
[4] ROKLIN L L. Magnesium alloy containing rare-earth metals: Structures and properties [M]. Taylor & Francis, 2002: 118.
[5] PURDY G R, KIRKALDY J S. Homogenization by diffusion [J]. Metall Trans, 1971, 2(2): 371-378.
[6] Y?CEL BIROL. The effect of homogenization practice on the microstructure of AA6063 billets [J]. Journal of Materials Processing Technology, 2004, 148: 250-258.
[7] JIA Z H, HU G Q, FORBORD B, SOLBERG J K. Effect of homogenization and alloying elements on recrystallization resistance of Al-Zr-Mn alloys [J]. Mater Sci Eng A, 2007, 444(1/2): 284-290.
[8] GAO Feng-hua, LI Nian-kui, TIAN Ni, SUN Qiang, LIU Xian-dong, ZHAO Gang. Overheating temperature of 7B04 high strength aluminum alloy [J]. Trans Nonferrous Met Soc China, 2008, 18(2): 321-326.
[9] MORDIKE B L, EBERT T. Magnesium properties applications potential [J]. Materials Science and Technology, 2001, A302(1): 37-45.
[10] GOURDET S, MONTHEILLET F. An experimental study of the deformation of a 304 type austenitic stainless steel [J]. Mater Sci Eng A, 1998, A255: 139-147.
[11] TAKUDA H, FUJIMOTO H, HATTA N. Modeling on flow stress of Mg-Al-Zn alloys at elevated temperatures [J]. Materials Processing Technology, 1998, 80/8l: 5l3-5l6.
[12] SEMIATIN S L, JONAS J J. Formability and workability of metals-plastic instability flow localization [M]. Ohio: ASM, Metals Park, 1984: 121-122.
[13] SHEPPARD T, PARSON N C, ZAIDI M A. Dynamic recrystallization in Al-7Mg [J]. Met Sci, 1983, 17(10): 481-490.
[14] ZENER C, HOLLOMON J H. Effect of strain rate upon the plastic flow of steel [J]. J Appl Phys, 1944, 15(1): 22-32.
[15] DUAN Yuan-pei, LI Ping. Flow behavior and microstructure evolution of TB8 alloy during hot deformation process [J]. Trans Nonferrous Met Soc China, 2007, 17: 1199-1204.
[16] LEE W S, LIN M T. The effects of strain rate and temperature on the compressive deformation behavior of Ti-6Al-4V alloy [J]. J Mater Process Technol, 1997, 167: 235-246.
[17] JONAS J J, SELLARS C M, McG W J. Strength and structure under hot-working conditions [J]. Int Metal Reviews, 1969, 3(1): 1-24.
[18] LUO Zi-jian, YANG Qi. New method to establish constitutive relationship considering effect of deformation heating [J]. The Chinese Journal of Nonferrous Metals, 2000, 10(6): 804-808. (in Chinese)
(Edited by YANG Bing)
Foundation item: Projects(2007CB613704, 2007CB613705) supported by the National Basic Research Program of China; Project(2006BAE04B01) supported by the National “Eleventh Five-Year Plan” Key Technologies R&D Program of China
Corresponding author: ZHANG Kui; Tel: +86-10-82241168; E-mail: zhkui@grinm.com


