- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 FT-IR spectra of BTR
- Fig.2 Effect of contact time on adsorption
- Fig.3 Relationship of -ln(1-F) and t for BTR
- Fig.4 Effect of pH value on adsorption
- Fig.5 Isotherms for adsorption of Ag(I) on BTR-1
- Fig.6 Langmuir isotherm for adsorption of Ag(I) on BTR-1
- Fig.7 Van’t Hoff plot for adsorption of Ag(I) on BTR-1
J. Cent. South Univ. Technol. (2011) 18: 361-366
DOI: 10.1007/s11771-011-0704-8![]()
Synthesis of 1,4-benzenedicarbonyl thiourea resins and their adsorption properties for Ag(I)
WANG Zhong-nan(王仲南)1, 2, ZHONG Hong(钟宏)1, WANG Shuai(王帅)1,
LIU Guang-yi(刘广义)1, ZHANG Qian(张骞)1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
2. College of Chemistry and Environmental Engineering, Shaoguan University, Shaoguan 512005, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract:
Several 1,4-benzenedicarbonyl thiourea resins (BTR) were synthesized through interfacial polymerization between 1,4- benzenedicarbonyl diisothiocyanate and polyamine. Their structures were confirmed by FT-IR. The adsorption properties (including the effect of adsorption time, pH, initial concentrations and temperature) of BTR-1, BTR-2 and BTR-3 for Ag(I) were investigated by batch tests. The results show that the adsorption equilibria of BTR-1, BTR-2, BTR-3 for Ag(I) are achieved after about 10 h. Their equilibrium adsorption capacities are 7.11, 6.75 and 6.23, respectively, and the adsorption process accords with G. E. Boyd equation and Langmuir adsorption isotherm as well. The adsorption capacities increase with the increase of pH (the highest uptake values are observed at pH being about 6-7). The thermodynamic parameters of BTR-1 were calculated. The results show that ?HΘ and ?SΘ are 6 958.8 J/mol and 64.28 J/(mol?K), respectively, and ?GΘ at 20, 30, 40 and 50 °C are -11.79, -12.52, -13.16 and -13.8 kJ/mol, respectively. The silver-loaded resins can be quantitatively eluted by a solution containing 6% thiourea in 1 mol/L HNO3.
Key words:
acylthiourea polymer; resin; interfacial polymerization; adsorption; Ag(I);
1 Introduction
Precious metals usually tend to form stable complexes with ligands containing “soft” donor atoms. As a donor containing N- and especially S-atom, thiourea is an excellent ligand to precious metal ions, which is widely used in the extraction, separation and recovery of precious metals. So, the investigations on the preparation and application of chelating resins with thiourea have been attracting much attention, and many research results had been reported [1-4]. Thiourea polymers reported were mainly obtained in two ways: one was polymer grafting, which meant to graft thiourea groups to the framework of polymers, such as polystyrene [4-5], polyvinyl chloride [6], poly(ethylene glycol) [7], phenolic aldehyde [8], chitosan [9], and silica gel [10]; the other was monomer polymerization, such as the polymerization of thiourea or bisthiourea and formaldehyde [1-2], or the polymerization of diisothiocyanate and diamine [11]. At present, most thiourea polymers reported are synthesized in the first way. Thiourea groups in these resins often locate in branches, and their adsorption performance is limited by the relatively low reaction efficiency and low content of thiourea groups. Contrarily, thiourea polymers obtained in the second way are reported less, in which thiourea groups locate in the main chain. With high content of thiourea group and ordered structure, they often show more excellent adsorption capacities and selectivity for precious metals. For example, in our previous works [12-14], some novel resins of poly(ester thiourea) were prepared by monomer polymerization. The adsorption capacities of ATR for Au(II) and Ag(I) are 4.65 and 4.40 mmol/g, respectively, and that of PETU for Ag(I) is 3.53 mmol/g.
In order to further research the adsorption properties for noble metals of these thiourea resins from the polymerization, in this work, several crosslinked acylthiourea polymers (1,4-benzenedicarbonyl thiourea resins or BTR) were synthesized through the interfacial polymerization of 1,4-benzendicarbonyl diisothiocyanate and polyamine. The synthesis process of BTR is more convenient and efficient than that of ATR and PETU. And their adsorption properties for Ag(I) were investigated.
2 Experimental
2.1 Reagent and apparatus
Metal nitrate or chloride was used as source of metal ions by dissolving exact nitrate or chloride in pure water. All chemicals are of analytical or chemical grade.
The infrared spectra were recorded on a G510PFTIR spectrophotometer. The adsorption experiments were carried out with a SHA-C thermostatic vibrator.
2.2 Synthesis of BTR resin
The synthesis of BTR included two steps. Firstly, 50 mmol ammonium thiocyanate (dried and triturated), 20 mmol 1,4-benzenedicarbonyl dichloride, about 75 mL methylene chloride (CH2Cl2) and 0.6 g PEG-400 were placed in a 250 mL flask equipped with a magnetic stirrer. The mixture was stirred for about 3 h at room temperature. The orange suspension was formed. Then, the reaction mixture was filtrated to remove the solvent. 1,4-benzendicarbonyl diisothiocyanate was obtained as orange solid. Secondly, in a 250 mL three-necked flask with mechanical stirrer, 10 mmol 1,4-benzenedicarbonyl diisothiocyanate dissolved in 50 mL CH2Cl2 reacted with 10 mmol diethylene triamine, or 8 mmol triethylene tetraamine or 6 mmol tetraethylene pentamine (respectively dissolved in about 80 mL water) for 1- 1.5 h at room temperature and with continuous stirring, and a yellow precipitate was formed. After being filtered, washed with water, ethanol and small quantity of ether successively, and then dried, several crosslinked acylthiourea polymers (BTR-1, BTR-2, BTR-3) were synthesized. The yields of BTR-1, BTR-2 and BTR-3 were 93.2%, 87.3% and 84.7%, respectively.
2.3 Adsorption procedure
2.3.1 Adsorption of BTR for metal ions
The adsorption behaviours of resins for Ag(I) and other metals were carried out by batch method. 50 mg BTR resin was placed into an iodometric flask containing 25 mL Ag(I) or other metal ions solution at an initial concentration of 0.1 mol/L. The pH was controlled by acetic acid/sodium acetate buffer or HNO3 or ammonia/ammonium chloride buffer. At desired temperature, the flask was shaken in the thermostatic vibrator at 150 r/min for a certain time. Then, the solution was filtrated off. The concentrations of Ag(I), Cu(II) and other metal ions in the solution were determined by Volhard method, iodimetry method and titration against EDTA, separately. Adsorption capacity could be calculated by
Q=(c0-ce)V/m (1)
where Q is the adsorption capacity (mmol/g), c0 is the initial concentration of solution (mol/L), ce is the concentration after adsorption (mol/L), V is the volume of solution (L), and m is the mass of the dry resin (g).
2.3.2 Regeneration of BTR
Elution experiments were also performed by batch method. A solution containing 1 mol/L HNO3 and 6% (mass fraction) thiourea was used as elution solution. After reaching the maximum uptake for Ag(I), the resin was filtrated and washed repeatedly. Then, the resin was suspended in elution solution in the iodometric flask. The flask was shaken for a certain time in the thermostatic vibrator. The adsorption-desorption operation was repeated several times, and the adsorption capacity of resin was recorded.
3 Results and discussion
3.1 Preparation of BTR
The synthesis route of BTR is represented as follows:
![]() (2)
(2)
![]()
![]()
 (3)
(3)
where for BTR-1, n=1; for BTR-2, n=2; for BTR-3, n=3.
FT-IR spectra of BTR are shown in Fig.1.
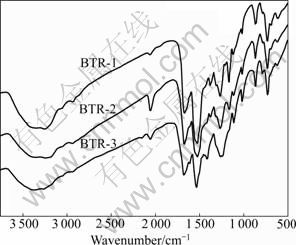
Fig.1 FT-IR spectra of BTR
The spectra show that N—H stretching vibration appears at 3 365-3 240 cm-1,C=O stretching vibration appears at about 1 674 cm-1, N—C—N stretching vibration appears at about 1 529 cm-1, and C=S vibration appears at about 1 263 and 1 109 cm-1. It is indicated that the resin contains functional groups of carbonyl and thiourea.
3.2 Adsorption capacities of BTR for metal ions
The adsorption experiments of BTR for Ag(I) and some metal ions were carried out under the following conditions: contact time 24 h, temperature 30 °C, initial concentration of metal ions 0.1 mol/L, and natural medium. The adsorption capacities for Ag(I), Cu(II), Zn(II), Ca(II), Mg(II), Fe(Ⅲ) are presented in Table 1. The obtained data display that for the same metal ion, the adsorption capacity order of BTR is BTR-1>BTR-2> BTR-3 basically.
Table 1 Adsorption capacities of BTR for metal ions
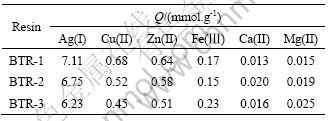
And it can also be seen that three BTRs have a high selectivity for Ag(I). This may be attributed to the strong affinity of silver towards sulphur as donor atom and the particular structure of resin. Owing to the conjugation among phenyl, carbonyl and thiourea, the softness of N and S donor atoms as soft bases is raised. According to the hard and soft acids and bases theory (HSAB), they will interact more strongly with soft acid,such as Ag(I), and on the other hand, they are weak to interact with borderline or hard acid,such as Cu(II), Zn(II), Fe(III), Ca(II) and Mg(II) .
3.3 Adsorption behaviours of BTR for Ag(I)
3.3.1 Effect of contact time on adsorption and adsorption kinetics
The effect of contact time on the adsorption of BTR for Ag(I) was studied at 30 °C, initial Ag(I) concentration of 0.1 mol/L, and pH value of 6.9. The results are presented in Fig.2.

Fig.2 Effect of contact time on adsorption
It can be seen that the adsorption capacities increase with the increase of contact time,and it is particularly rapid in the initial stage of 4 h. And after about 10 h, the adsorption equilibrium of BTR-1, BTR-2 and BTR-3 for Ag(I) is achieved. It is also shown that the adsorption rate of BTR for Ag(I) is slow. The adsorption data of Fig.2 are treated according to the Boyd diffusion equation of liquid film:
-ln(1-F)=kt (4)
F is obtained by the expression F=Q/Qm, where Q and Qm refer to the amounts (mmol/g) of Ag(I) absorbed at time t (h) and at equilibrium, respectively, and k is the overall rate constant (h-1).
The obtained results are presented in Fig.3 and Table 2.
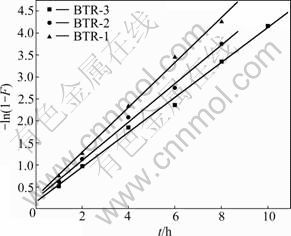
Fig.3 Relationship of -ln(1-F) and t for BTR
Table 2 Linear equations of adsorption kinetics of BTR

It is obvious that a linear relationship exists between -ln(1-F) and t. The rate constant k is 0.397, 0.437 and 0.509 h-1, respectively. This also reflects that the adsorption rate order of BTR for Ag(I) is BTR-3> BTR-2>BTR-1. This may have relationship with the different lengths of main chain in BTR-3, BTR-2 and BTR-1. It is suggested that the kinetics of adsorption process of BTR is controlled by the diffusion through the liquid film surrounding the solid adsorbents.
3.3.2 Effect of pH on adsorption
Adsorption experiments under controlled pH were carried out under the conditions that the contact time is 10 h, the adsorption temperature is 30 °C, and the initial concentration of Ag(I) is 0.1 mol/L. Considering the hydrolysis of Ag(I), the highest pH value selected is 6.9 (natural). The results are shown in Fig.4.
It can be seen that the adsorption capacities increase with the increase of pH value. The highest uptake values are observed at pH of about 6-7. The observed result that the uptake values decrease with pH value decreasing may be attributed to the partial protonation of the amino group and thiourea group [15]. This hinders the interaction of the resins and Ag(I) ion.
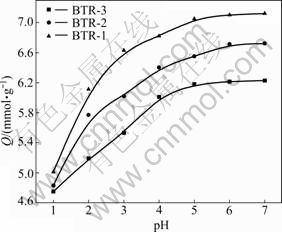
Fig.4 Effect of pH value on adsorption
3.3.3 Effect of initial concentration and temperature on adsorption and isotherm
BTR-1 was selected to study the effect of initial concentration of Ag(I) and temperature on the adsorption and isotherm. The solution was controlled at pH=6.9. The results are presented in Fig.5.
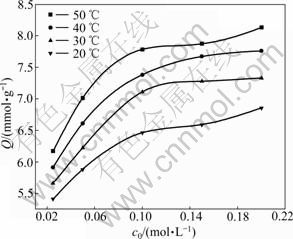
Fig.5 Isotherms for adsorption of Ag(I) on BTR-1
The adsorption curves indicate that the adsorption capacity for Ag(I) increases with the increase of the
initial concentration (c0) of Ag(I) at the same temperature, but increases slowly and is gradually close to the maximum uptake when c0 exceeds 0.1 mol/L. On the other hand, at the same initial concentration (c0), the adsorption capacity increases with the rise of the temperature. The effect of temperature on the process can be explained by two reasons: 1) increasing the active sites available for the interaction with the rise of temperature [16], and 2) rising temperature leading to higher dehydration of Ag(I) and consequently their interaction becoming more favorable [17].
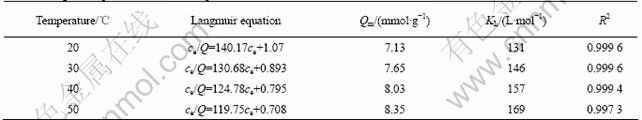
The adsorption data are plotted according to Langmuir equation:
ce/Q=ce/Qm+1/QmKL (5)
where ce is the equilibrium concentration of metal ions in solution (mol/L), Qe is the absorbed value of metal ions at equilibrium concentration (mmol/g), Qm is the maximum adsorption capacity (mmol/g), and KL (L/mol) is the Langmuir constant which is related to the energy of adsorption. The results are presented in Fig.6 and Table 3.
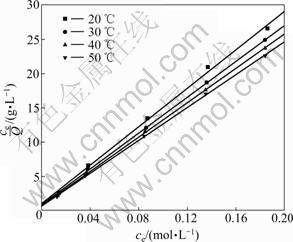
Fig.6 Langmuir isotherm for adsorption of Ag(I) on BTR-1
It can be seen that the plot of ce/Q vs ce gives a straight line with the correlation coefficient exceeding 0.99. This implies that the adsorption process of Ag(I) on BTR can be described by Langmuir equation.
3.4 Adsorption thermodynamics of Ag(I)
Thermodynamic parameters could be calculated from Van’t Hoff equation [15-16]:
ln KL=-?HΘ/RT+?SΘ/R (5)
where ?HΘ and ?SΘ are enthalpy and entropy changes, respectively, R is the universal gas constant (8.314 J/(mol?K)) and T is the absolute temperature (K).
Table 3 Langmuir equation of BTR-1 and parameters
The plot of ln KL against 1/T gives a straight line with the slope equal to -?HΘ/RT and the intercept equal to ?SΘ/R. The results are presented in Fig.7 and Table 4.
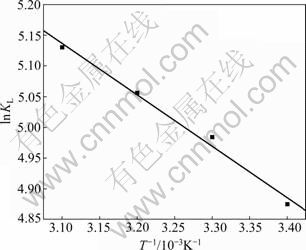
Fig.7 Van’t Hoff plot for adsorption of Ag(I) on BTR-1
Table 4 Results of Van’t Hoff plots and thermodynamic parameters

The positive values of ?HΘ and ?SΘ refer to an endothermic adsorption process and an increase in entropy. The observed increase in negative values of ?GΘ with increasing the temperature implies also that the adsorption becomes more favorable at higher temperature. And the adsorption process is dominated by entropic rather than enthalpy changes.
3.5 Regeneration of BTR
The regeneration experiments were performed with silver-loaded BTR-1 at 30 °C using 6% thiourea solution containing 1 mol/L HNO3 as eluent. The adsorption– desorption operation was repeated six times. The results are listed in Table 4. The capacity after regeneration remains 88% that of the first time. This indicates that BTR-1 resin has better regeneration property.
Table 5 Regeneration properties of BTR-1
![]()
4 Conclusions
1) A series of novel 1,4-benzendicarbonyl thiourea chelating resins (BTR) were synthesized through the interfacial polymerization between 1,4- benzenedicarbonyl diisothiocyanate and polyamine. The synthesis process is more convenient and efficient. The yields of BTR-1, BTR-2 and BTR-3 are 93.2%, 87.3% and 84.7%, respectively.
2) The equilibrium adsorption capacities of BTR-1, BTR-2, BTR-3 for Ag(I) (contact time 24 h, temperature 30 °C, initial concentration of metal ions 0.1 mol/L, and natural medium) are 7.11, 6.75, 6.23 mmol/g, respectively. The adsorption capacity order of BTR is BTR-1>BTR-2>BTR-3.
3) The adsorption process for Ag(I) is found in accord with Boyd equation and Langmuir adsorption isotherm as well. The obtained thermodynamic parameters show that the adsorption process is spontaneous and dominated by entropy change rather than enthalpy change, and the adsorption becomes more favorable at higher temperatures.
4) The silver-loaded resins can be quantitatively eluted by a solution containing 6% thiourea in 1 mol/L HNO3.
References
[1] NI Cai-hua, YI Chang-hai, FENG Zhi-yun. Studies of syntheses and adsorption properties of chelating resin from thiourea and formaldehyde [J]. Journal of Applied Polymer Science, 2001, 82(13): 3127-3132.
[2] ATIA A A. Adsorption of silver and gold on resin derived from bisthiourea and application to retrieval of silver ion from processed photo film [J]. Hydrometallurgy, 2005, 80(1/2): 98-106.
[3] XU Yu-wu, YANG Ya-he, LI Han-ping. Study on chelating resins xxx: Syntheses and adsorption properties of thiourea type resins [J]. Journal of Wuhan University: Natural Science Edition, 1999, 45(2): 129-134. (in Chinese)
[4] ZUO G J, MUHAMMED M. Thiourea-based coordinating polymers: Synthesis and binding to noble metals [J]. Reactive Polymers, 1995, 24(3): 165-181.
[5] TROCHIMCZUK A W, KOLARZ B N. Synthesis and chelating properties of resin with methylthiourea, guanylthiourea and dithiocarbamate groups [J]. European Polymer Journal, 2000, 36(11): 2359-2363.
[6] CAO De-rong, SU Zhi-xing. Chemical conversion for macroporous spherical resins of PVC(III): Studies on the synthesis and properties of thiourea resins [J]. Journal of Lanzhou University: Natural Science Edition, 1997, 33(2): 63-67. (in Chinese)
[7] YANG Gui-chun, CHEN Zu-xing, ZHANG Zhao-jun. Combinatorial synthesis of novel thiourea derivatives on a modified poly(ethyleneglycol) [J]. Reactive and Functional Polymers, 2002, 51(1): 1-6.
[8] LIU Chun-ping, SUN Lin, LU Ju-bo, LIU Bing, QU Rong-jun. Adsorption properties and laboratory use of phenylthiourea supported phenolic aldehyde chelating resin for Ag+ [J]. Ion Exchange and Adsorption, 2005, 21(4): 335-342. (in Chinese)
[9] PENG Chang-hong, CHEN Yi-feng, TANG Mo-tang. Synthesis and adsorption properties of chitosan-crown ether resins [J]. Journal of Central South University of Technology, 2003, 10(2): 103-107.
[10] LIU Peng, SU Zhi-xing, WU Xiong-zhi, PU Qiao-sheng. Application of isodiphenylthiourea immobilized silica gel to flow injection on-line microcolumn preconcentration and separation coupled with flame atomic absorption spectrometry for interference determination of trace silver, gold, palladium and platinum in geological and metallurgical samples [J]. Journal of Analytical Atomic Spectrometry, 2002, 17(7): 125-130.
[11] MAO Xue-pu, HANG Feng-jin, DUAN Zhi-fang. Synthesis and characterization of novel multifunctional acylthiourea polymers [J]. Chinese Chemical Letters, 2005, 16(5): 609-612.
[12] WANG Shuai, ZHONG Hong, LIU Guang-yi, ZHANG Qian, LI Ting. Synthesis and adsorption properties for Au(III) of alkoxycarbonyl thiourea resin [J]. Journal of Central South University of Technology, 2008, 15(4): 463-468.
[13] ZHONG Hong, WANG Shuai, QIU Yun-ren, WANG Ai-ping. Synthesis of chelating resin PETU and its adsorption to Ag(I) [J]. The Chinese Journal of Process Engineering, 2007, 7(4): 689-693.
[14] WANG Shuai, ZHONG Hong, WANG Ai-ping. Synthesis of thiourea resins and their adsorption for metal ions [J]. Chinese Journal of Applied Chemistry, 2007, 24(8): 941-944. (in Chinese)
[15] DONIA A M, ATIA A A, EL-BORAEY H A, Adsorption of Ag(I) on glycidyl methacrylate/N,N’-methylene bis-acrylamide chelating resins with embedded iron oxide [J]. Separation and Purification Technology, 2006, 48: 281-287.
[16] BOONAMNUAYVITAYA V, CHAIYA C, TANTHAPICHAKOON W, JARUDILKKUL S. Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay [J]. Separation and Purification Technology, 2004, 35(1): 11-22.
[17] TAHIR S S, RAUF N. Thermodynamic studies of Ni(II) adsorption onto bentonite from aqueous solution [J]. Journal of Chemical Thermodynamics, 2003, 35(12): 2003-2009.
(Edited by YANG Bing)
Foundation item: Projects(20476105, 50604016) supported by the National Natural Science Foundation of China
Received date: 2010-03-08; Accepted date: 2010-10-11
Corresponding author: ZHONG Hong, Professor, PhD; Tel: +86-731-88830603; E-mail: zhongh@mail.csu.edu.cn
- Synthesis of 1,4-benzenedicarbonyl thiourea resins andtheir adsorption properties for Ag(I)


