
Photoelectrocatalytic activity of two antimony doped SnO2 films for
oxidation of phenol pollutants
WANG Yan(王 艳)1, 2, FAN Cai-mei(樊彩梅)1, HUA Bo(华 勃)1,
LIANG Zhen-hai(梁镇海)1, SUN Yan-pin(孙彦平)1
1. College of Chemistry and Chemical Engineering, Taiyuan University of Technology,
Taiyuan 030024, China;
2. College of Chemistry and Bioengineering, Taiyuan University of Science and Technology,
Taiyuan 030021, China
Received 3 July 2008; accepted 8 November 2008
Abstract:
Two types of Sb-doped SnO2 films on titanium substrate were prepared by the combination of electro-deposition and dip-coating (Ti/SnO2-Sb2O4/SnO2-Sb2O4) and single dip-coating (Ti/SnO2-Sb2O4), respectively. The surface morphology and crystalline structure of both film electrodes were characterized using X-ray diffractometry(XRD) and scanning electron microscopy(SEM). XRD spectra indicate that the rutile SnO2 forms in two films and a TiO2 crystallite exists only in Ti/SnO2-Sb2O4 electrode. SEM images show that the surface morphology of two films is typically cracked-mud structure. The photooxidation experiment was proceeded to further confirm the two electrode activity. The results show that the photoelectrocatalytic degradation efficiency of Ti/SnO2-Sb2O4 electrode with sub-layer is higher than that of simple Ti/SnO2-Sb2O4 electrode using phenol as a model organic pollutant. The Ti/SnO2-Sb2O4/SnO2-Sb2O4 photoanode has a better photoelectrochemical performance than Ti/SnO2-Sb2O4 photoanode for the removal of organic pollutants from water.
Key words:
Ti/SnO2-Sb2O4; SnO2 films; photoelectrocatalysis; phenol;
1 Introduction
Photoelectrocatalytic process using semiconductor electrodes has been widely studied due to their inherent high catalytic activity for oxidation of organic pollutants in the past two decades[1-3]. Especially, metal/TiO2 photoelectrode has received a lot of attention, because this technology can suppress the recombination of photogenerated electron-hole pairs by external bias[4-5], and solve the separation problem of TiO2 particles from water solution. Unfortunately, applying external bias to Ti/TiO2 electrode could improve the photocatalytic activity of Ti/TiO2 electrode[6-8], but the improvement is very limited. The Ti/SnO2 film electrode has attracted a lot of attention due to its high oxygen–evolution overpotential in electrochemical process[9-10]. Our study about Ti/SnO2 film electrode allowed effective photooxidation of phenol under UV irradiation[11]. Thus we designed the Ti/SnO2 as a novel photoanode in the treatment of organics in water by photoelectrocatalysis. However, its poor conductivity limited its catalytic activity for oxidation of organics in water[12-14]. One of the methods to increase the electricity conductivity is to modify SnO2 by doping various elements, such as F, P and Sb[15-17]. Among these elements, Sb-doped SnO2 showed good electrical conductivity, high overpotential for oxygen–evolution and catalytic activity. On the other hand, Sb-doped SnO2 is still a semiconductor (Eg= 3.5-3.8 eV) and has the function of photoanode, although its application in the photoelectrocatalytic process has not been noted as TiO2 thin film anode.
Besides, the preparation technology and method of electrode have much important effect on electrode function and performance as well as service life. Recently, many papers have reported the preparation and properties of SnO2 film, such as sol-gel dip coating [18-19], spray pyrolysis[20-21], sputtering[22-23], chemical vapor deposition[24-25], and thermal evapora- tion[26]. Although various studies have demonstrated the dependence of the properties of Sb-doped SnO2 on preparation condition and method, there is little information about the performance of these modified SnO2 as photoelectrocatalysts for the oxidation of organic pollutants.
In this work, two electrodes were prepared: one was coated directly with catalyst layer (SnO2-Sb2O4) to titanium substrate surface to form Ti/SnO2-Sb2O4 electrode; the other included a SnO2-Sb2O4 sub-layer to form Ti/SnO2-Sb2O4/SnO2-Sb2O4 electrode. Phenol was selected as model compound for study of catalytic performance of Sb-doped SnO2 photoelectrocatalysis. Our goals are to evaluate the influences of Sb modifier and SnO2-Sb2O4 sub-layer on electrode microstructure, electrochemical properties and photoelectrocatalytic activity for phenol oxidation.
2 Experimental
2.1 Preparation of Sb-doped SnO2 electrode
2.1.1 Pre-treatment of Ti substrate
Ti plates with size of 70 mm×10 mm were polished using abrasive paper prior to etch in 40% NaOH solution at 95 ℃ for 4 h. Additional acid etching was carried out using 10% oxalic acid solution at the same condition. The etched Ti plate was then washed thoroughly using deionized water and dried.
2.1.2 Preparation of Ti/SnO2-Sb2O4/SnO2-Sb2O4 anode
The anode was prepared in two steps. First, SnO2-Sb2O4 sub-layer was prepared using electro- deposition technique. In this process, the pretreated Ti plate was used as cathode and insoluble electrode as anode with 100 mL electrolyte solution containing 1.753 g SnCl4·4H2O, 0.114 g SbCl3, and 12 mL ethanol solution (analytical reagent) of tetrabutyl titanate (analytical reagent). A constant DC current of 0.12 A was applied for 30 min to electroplate the cathode, and then the Ti plate with electroplated sub-layer was dried in an oven at 450 ℃ for 2 h. Subsequent loading of the second layer of SnO2-Sb2O4 was accomplished using dip-coating technique. The coating solution was made using 4 g SnCl4·5H2O, 0.156 g SbCl3 and 1 mL concentrated HCl (37%) in 9 mL n-butanol (analytical reagent). After washing coating, the sample was dried at 100 ℃ for 5 min prior to calcination at 450 ℃ for 10 min. The dipping and thermal deposition process, washing coating and calcination process were repeated several times until reaching the loading times. The resulting electrode was annealed at 450 ℃ for 1 h to form Ti/ SnO2-Sb2O4/SnO2-Sb2O4 electrode.
2.1.3 Preparation of Ti/SnO2-Sb2O4 anode
The anode was prepared using dip-coating procedure as described above. The final electrode was also called Ti/SnO2-Sb2O4 electrode without sub-layer.
2.2 Preparation of gas diffusion electrode
The gas diffusion electrode was prepared using coating and pressing method. Graphite, carbon black and nickel mesh were pressed to about 1 mm in thickness and annealed at 400 ℃ for 1 h. The resulting electrode was used as cathode for photoelectrocatalysis(PEC) process. Detailed preparing process can be found in Ref.[27].
2.3 Analytical methods
Scanning electron microscope(SEM) (LEO-438VP, Japan) equipped with an energy-disperse spectrometer (EDS) analyzer was used to study the surface morphology. X-ray diffraction (XRD) (D/max-2500, Japan) measurements were carried out to determine crystal phase structure of Ti/SnO2-Sb2O4 electrode. UV-visible spectrophotometer (Model Cary50, USA) was used to measure phenol concentration. Total organic carbon(TOC) of phenol containing solutions was measured using a TOC analyzer (Multi N/C 3000, Germany).
2.4 Photoelectrocatalytic reactor and experimental procedure
Fig.1 shows a schematic diagram of the batch scale experimental reactor system. The photoreactor system consists of a cylindrical quartz glass reactor with an effective vessel volume of 100 mL, an external UV light source and a two-electrode configuration. The two electrodes were placed in the reactor parallelly. Ti/SnO2-Sb2O4 electrode faced to UV light was used as photocatalyst and electroanode. The gas diffusion electrode was used as cathode. The applied potential bias was obtained from a DC potentiostat (model 363, England). A 250 W high-pressure mercury lamp (main wavelength 365 nm, China) with a cooling water quartz jacket acted as a side light source. Air was supplied to the reaction system from the bottom of reactor using a mini-type air pump.
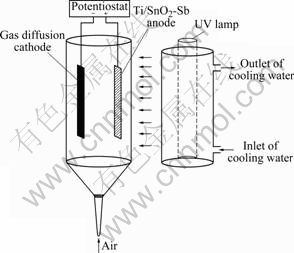
Fig.1 Schematic diagram of photoreaction systems
During the reaction, phenol solution with the initial content of 20 mg/L was irradiated by UV light and aerated by air. Samples of phenol solution were taken periodically for measuring both phenol and TOC contents.
3 Results and discussion
3.1 XRD analysis
No apparent change was found in the XRD pattern of the Ti/SnO2-Sb2O4/SnO2-Sb2O4 electrode before and after use (Fig.2). In contrast, a TiO2 peak is observed for Ti/SnO2-Sb2O4 electrode without sub-layer of SnO2-Sb2O4 after 30 h. This indicates the oxidation of Ti substrate during reaction process. Ti oxidation may further result in weak conductivity of Ti/SnO2-Sb2O4 electrode[28]. Applying a sub-layer to Ti surface is proved to be useful in preventing Ti from oxidation and improving photoelectrocatalytic activity of Sb-doped SnO2 electrode.

Fig.2 XRD patterns of Ti/SnO2-Sb2O4 electrodes: (a) With sub-layer before use; (b) With sub-layer after use of 30 h; (c) Without sub-layer before use; (d) Without sub-layers after use of 30 h
3.2 SEM analyses
The surface morphologies of the Sb-doped SnO2 electrodes are shown in Fig.3. Fig.3(a) reveals a roughly porous surface of the Ti substrate after pretreatment. Roughness of the pretreated Ti surface is critical for generating desired catalysts layer on Ti surface. A flat and dense surface is formed after sub-layer of SnO2- Sb2O4, which is applied by electro-deposition process(Fig.3(b)). It is believed that this dense surface provides barrier for oxygen diffusion and reaction with Ti as evidence by XRD study above. Both the surfaces of the Ti/SnO2-Sb2O4 electrode prepared by dip-coating procedure (Fig.3(c)) and the double layered Ti/SnO2-Sb2O4/SnO2-Sb2O4 electrode by a procedure of electro-deposition and subsequent dip-coating (Fig.3(d)) look rather rough with a mixed mud-flat cracking and agglomerates. The factor leading to the formation of such a complex morphology may be the difference in thermal expansion coefficients of substrates or layer materials[29]. The surface morphology significantly influences the activity of the electrode for the oxidation of phenol in aqueous solution. Lower activity of Ti/SnO2-Sb2O4 electrode without sub-layer is apparently related to cracks which may provide convenience for oxygenous infiltration and diffusion, resulting in the decrease of electrode performance. Thus, adding sub-layer is an appropriate measure for the preparation of Ti/SnO2-Sb2O4 electrode.
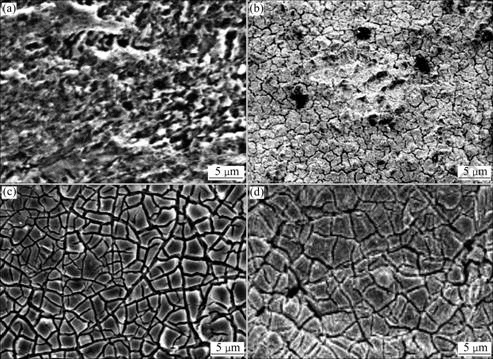
Fig.3 SEM images of Ti/SnO2-Sb2O4 electrodes
Furthermore, both the Ti/SnO2-Sb2O4 and Ti/SnO2-Sb2O4/SnO2-Sb2O4 electrodes were analyzed by means of EDS, and the results about the molar ratios of Sn and Sb are shown in Table 1. In this study, the theoretical composition ratio of Sn to Sb atoms is 100:6. From the results of Table 1, it can be found that the molar ratios of the final coatings are not consistent with the theoretical composition. There are two reasons: one is element volatilization during heat treatment, and the other is instrument error by itself.
Table 1 Composition of Ti/SnO2-Sb electrode (molar fraction, %)

3.3 Performance of phenol degradation
Complete phenol oxidation was achieved using the Ti/SnO2-Sb2O4/SnO2-Sb2O4 electrode after 2 h reaction, while only about 86% phenol conversion was achieved under the same conditions using Ti/SnO2-Sb2O4 electrode (Fig.4). The phenol mineralization rates after 3 h were determined to be 85% and 73% for Ti/SnO2- Sb2O4/SnO2-Sb2O4 electrode and Ti/SnO2-Sb2O4, respectively. These results indicate that the sub-layer of SnO2-Sb2O4 on Ti substance improves the activity of the catalysts for phenol oxidation.

Fig.4 Effect of sub-layer on photoelectrocatalytic degradation of phenol
3.4 Linear voltammogram
The current properties of the Ti/SnO2-Sb2O4/ SnO2-Sb2O4 and Ti/SnO2-Sb2O4 electrodes were investigated in phenol solution without electrolyte, and the linear voltammograms of two electrodes are shown in Fig.5. In dark, the anode current of the Ti/SnO2-Sb2O4/ SnO2-Sb2O4 electrode is higher than that of the Ti/SnO2-Sb2O4 on the condition of only anodic bias, which is similar to the result of the combination of the same anodic bias and UV illumination. Under UV illumination, the current efficiency of the two electrodes is better than that in dark, and the Ti/SnO2-Sb2O4 electrode with sub-layer can acquire a higher photocurrent[30] than Ti/SnO2-Sb2O4 electrode without sub-layer at the same anode bias, which indicates that the Ti/SnO2-Sb2O4/ SnO2-Sb2O4 electrode can promote the separation of photoelectrocatalytic electron-hole pair due to improved anode conductivity. Moreover, the probability of electrons that can get away from hole recombination increases with increasing the voltage. As a result, it is necessary to employ an anodic bias in photocatalytic process.

Fig.5 Linear voltammograms of Ti/SnO2-Sb2O4 electrode with sub-layer and without sub-layer (scanning speed: 100 mV/s, scanning range: -2.0-2.0 V (vs SCE))
3.5 Comparison of different degradation processes
The efficiencies of phenol degradation and TOC degradation from phenol solution were measured at four different processes, which were adsorption, photocatalytic, electrocatalytic and photoelectrocatalytic processes, and the results are presented in Fig.6. During all the experiments, the Ti/SnO2-Sb2O4/SnO2-Sb2O4 electrode and a gas diffusion electrode were immersed in the phenol solution, and air was supplied to the reaction system from the bottom of reactor.
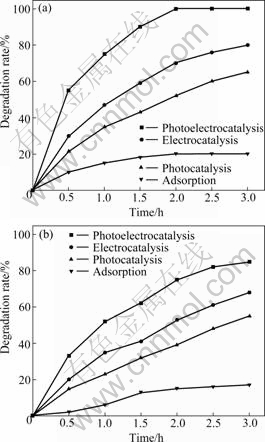
Fig.6 Comparison of removal rate of different degradation processes: (a) Phenol removal; (b) TOC removal
The adsorption effect of phenol plays an important role in the phenol removal, because the adsorption of organic pollutants on the catalysts is the key step in the catalytic reaction[31]. Thus, the adsorption effect of the two electrodes must be considered at first. Adsorption process was performed in the absence of the UV light and potentiostat for 3 h, and only 20% of phenol was removed. For the photocatalytic degradation process alone, the phenol degradation rate can reach 65% after 3 h reaction. When 2.0 V cell voltage was applied for 3 h in the absence of UV illumination, the phenol degradation rate was 80%. However, both in the presence of 2.0 V cell voltages and UV illumination, the phenol concentrations were decreased rapidly with increasing reaction time, and the degradation rate can reach 100% after 2 h. It can be seen that the phenol removal increases with the increase of reaction time in all four processes, but the photoelectrocatalytic degradation rate was obviously higher than that of other processes. Thus, it can be concluded that phenol can be removed from the water more efficiently by photoelectrocatalytic process.
A similar conclusion can be drawn by comparison of the TOC reduction efficiency, which is shown in Fig.6(b). TOC removal efficiencies are 17%, 68% and 55% in the presence of adsorption, photocatalytic and electrocatalytic processes, respectively, while the phenol mineralization rate of photoelectrocatalytic process can reach 85%. The TOC removal rate in the photoelectrocatalytic process is the highest. This should be attributed to the synergetic effect between photocatalysis and electrocatalysis in this photoreactor, and the adsorption also plays an important role in the degradation process.
4 Conclusions
1) Two electrodes of Ti/SnO2-Sb2O4/SnO2-Sb2O4 and Ti/SnO2-Sb2O4 were obtained. The Ti/SnO2-Sb2O4 was prepared by the simple procedure of dip-coating method and the Ti/SnO2-Sb2O4/SnO2-Sb2O4, i.e. the Sb-doped SnO2 film electrode with Sb-doped SnO2 film sub-layer, was prepared by the procedure of electro- deposition technique and subsequent dip-coating method.
2) The XRD spectra of two film electrodes (Ti/SnO2-Sb2O4/SnO2-Sb2O4 and Ti/SnO2-Sb2O4), which were annealed at 450 ℃, correspond to rutile SnO2, and an anatase TiO2 crystallite is found only in the thin film of Ti/SnO2-Sb2O4 electrode. SEM measurement shows that Ti/SnO2-Sb2O4/SnO2-Sb2O4 electrode looks rougher with a mixed mud-flat cracking and agglomerates.
3) The Ti/SnO2-Sb2O4/SnO2-Sb2O4 electrode can acquire a higher photocurrent than Ti/SnO2-Sb2O4 electrode.
4) The degradation experiments show that the Sb-doped SnO2 film electrode with sub-layer has a higher phenol and TOC removal rate than Ti/SnO2-Sb2O4 electrode in electrocatalytic process and photo- electrocatalytic process. Therefore, Ti/SnO2-Sb2O4/ SnO2-Sb2O4 is not only a good electrochemical anode in treating organic pollutants but also a good photoanode in electro-assisted photocatalytic process for complete oxidation of organic pollutants.
References
[1] WALDNER G, POURMODJIB M, BAUER R, NEUMANN- SPALLART M. Photoelectrocatalytic degradation of 4-chlorophenol and oxalic acid on titanium dioxide electrodes [J]. Chemosphere, 2003, 50(8): 989-998.
[2] CHENG Xiao-fang, LENG Wen-hua, PI Ou-yang, ZHANG Zhao, ZHANG Jian-qing, CAO Chu-nan. Enhancement of photocatalytic activity of TiO2 film electrode by in situ photoelectro-generating active chlorine [J]. Trans Nonferrous Met Soc China, 2007, 17(5): 1087-1092.
[3] RADECKA M. TiO2 for photoelectrolytic decomposition of water [J]. Thin Solid Films, 2004, 451/452(22): 98-104.
[4] WALKER S A, CHRISTENSEN P A, SHAW K E. Photoelectrochemical oxidation of aqueous phenol using titanium dioxide aerogel [J]. Journal of Electroanalytical Chemistry, 1995, 393(1/2): 137-140.
[5] ZLAMAL M, MACAK J M, SCHMUKI P, KR?SA J. Electrochemically assisted photocatalysis on self-organized TiO2 nanotubes [J]. Electrochemistry Communications, 2007, 9(12): 2822-2826.
[6] ZANONI M V B, SENE J J, ANDERSON M A. Photoelectrocatalytic degradation of Remazol Brilliant Orange 3R on titanium dioxide thin-film electrodes [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2003, 157(1): 55-63.
[7] YANG Shao-gui, LIU Ya-zi, SUN Ceng. Preparation of anatase TiO2/Ti nanotube-like electrodes and their high photoelectrocatalytic activity for the degradation of PCP in aqueous solution [J]. Applied Catalysis A: General, 2006, 301(2): 284-291.
[8] QUAN Xie, CHEN Shuo, SU Jing, CHEN Jing-wen, CHEN Guo-hua. Synergetic degradation of 2, 4-D by integrated photo and electrochemical catalysis on a Pt doped TiO2/Ti electrode [J]. Separation and Purification Technology, 2004, 34(1/3): 73-79.
[9] GRIMM J, BESSARABOV D, MAIER W, STORCK S, SANDERSON R D. Sol-gel film preparation of novel electrodes for the electrocatalytic oxidation of organic pollutants in water [J]. Desalination, 1998, 115(3): 295-302.
[10] ZHANG Q S, WU H H. The research on electrocatalytic oxidation of phenol using the SnO2/Ti electrode by thermal oxidation [J]. Electrochemistry, 1999, 5: 401-405.
[11] ZHANG Xiao-yan, FAN Cai-mei, LIANG Zhen-hai, SUN Yan-pin. Synergetic effect of oxygen diffusion electrode and Ti/SnO2/Sb2O4 electrode in photoelectrocatalytic degradation of phenol in water [J]. Journal of Taiyuan University of Technology, 2007, 38(2): 130-132. (in Chinese)
[12] EPIFANI M, ALVISI M, MIRENGHI L. Sol-gel processing and characterization of pure and metal-doped SnO2 thin films [J]. Journal of the American Ceramic Society, 2001, 84: 48-54.
[13] CANUT B, TEODORESCU V, ROGER J A, BLANCHIN M G, DAOUDI K, SANDU C. Radiation-induced densification of sol-gel SnO2:Sb films [J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2002, 191(1/4): 783-788.
[14] DAL SANTOS M A, ANTUNES A C, RIBEIRO C, BORGES C P F, ANTUNES S R M, ZARA A J, PIANARO S A. Electric and morphologic properties of SnO2 films prepared by modified sol-gel process [J]. Materials Letters, 2003, 57(28): 4378-4381.
[15] HU Y, HOU S H. Preparation and charaterization of Sb-doped SnO2 thin films from colloidal precursors [J]. Materials Chemistry and Physics, 2004, 86(1): 21-25.
[16] ZHANG Cun-zhong, LIU Zhen, WU Feng, LIN Li-jun, QI Fei. Electrochemical generation of ferrate on SnO2-Sb2O3/Ti electrodes in strong concentration basic condition [J]. Electrochemistry Communications, 2004, 6(11): 1104-1109.
[17] LEE S Y, PARK B O. Structural, electrical and optical characteristics of SnO2:Sb thin films by ultrasonic spray pyrolysis [J]. Thin Solid Films, 2006, 510(1/2): 154-158.
[18] GRIMM J H, BESSARABOV D G, SIMON U. Characterization of doped tin dioxide anodes prepared by a sol-gel technique and their application in an SPE-reactor [J]. Journal of Applied Electrochemistry, 2000, 30: 293-302.
[19] ZHANG Jian-rong, GAO Lian. Synthesis and characterization of nanocrystalline tin oxide by sol-gel method [J]. Journal of Solid State Chemistry, 2004, 177: 1425-1430.
[20] CORREA-LOZANO B, COMNINELLIS C, DE BATTISTI A. Electrochemical properties of Ti/SnO2-Sb2O5 electrodes prepared by the spray pyrolysis technique [J]. Journal of Applied Electrochemistry, 1996, 26: 683-688.
[21] PARAGUAY-DELGADO F, MIKI-YOSHIDA M, ANTUNEZ W, GONZ?LEZ-HERN?NDEZ J, VOROBIEW Y V, PROKHOROV E. Morphology and microstructure of textured SnO2 thin films obtained by spray pyrolysis and their effect on electrical and optical properties [J]. Thin Solid Films, 2008, 516(6): 1104-1111.
[22] JAEHYEONG L. Effects of oxygen concentration on the properties of sputtered SnO2:Sb films deposited at low temperature [J]. Thin Solid Films, 2008, 516(7): 1386-1390.
[23] GODFROID T, GOUTTEBARON R, DAUCHOT J P, LECL?RE P, LAZZARONI R, HECQ M. Growth of ultrathin Ti films deposited on SnO2 by magnetron sputtering [J]. Thin Solid Films, 2003, 437(1/2): 57-62.
[24] DUVERNEUIL P, MAURY F, PEBERE N, SENOCQ F, VERGNES H. Chemical vapor deposition of SnO2 coatings on Ti plates for the preparation of electrocatalytic anodes [J]. Surface and Coatings Technology, 2002, 151: 9-13.
[25] AMJOUD M B, MAURY F, SOUKANE S, DUVERNEUIL P. Making of specific electrodes by CVD [J]. Surface and Coatings Technology, 1998, 100/101: 169-172.
[26] STRY?HAL Z, PAVL?K J, NOV?K S, MACKOV? A, PE?INA V, VELTRUSK? K. Investigations of SnO2 thin films prepared by plasma oxidation [J]. Vacuum, 2002, 67(3/4): 665-671.
[27] FAN Cai-mei, ZHANG Xiao-yan, WANG Yun-fang, HAO Xiao-gang, SUN Yan-ping. Properties of oxygen electrodes in photoelectrocatalytic degradation of phenol in water [J]. Chinese Journal of Applied Chemistry, 2007, 24(4): 389-391.
[28] CORREA-LOZANO B, COMNINELLIS C, DE BATTISTI A. Service life of Ti/SnO2-Sb2O5 anodes [J]. Journal of Applied Electrochemistry, 1997, 27: 970-974.
[29] CUI Yu-hong, FENG Yu-jie, LIU Jun-feng. Preparation and characterization on Sb doped Ti-base SnO2 electrocatalytic electrodes [J]. Function Material, 2005, 36(2): 234-237. (in Chinese)
[30] FENG Yu-jie, CUI Yu-hong, WANG Jian-jun. Preparation and characterization of Dy doped Ti-base SnO2/Sb electrocatalytic electrodes [J]. Chinese Journal of Inorganic Chemistry, 2005, 21(6): 836-841. (in Chinese)
[31] HE Chun, XIONG Ya, SHU Dong, ZHU Xi-hai, LI Xiang-zhong. Preparation and photoelectrocatalytic activity of Pt (TiO2)-TiO2 hybrid films [J]. Thin Solid Films, 2006, 503: 01-07.
Foundation item: Projects(20476070,20876104) supported by the National Natural Science Foundation of China
Corresponding author: FAN Cai-mei; Tel: +86-351-6018193; E-mail: fancm@163.com
DOI: 10.1016/S1003-6326(08)60349-0
(Edited by YUAN Sai-qian)


