
Effects of precipitation strengthening heat treatment for Al-Mg alloy
Seong-Jong KIM1, Seok-Ki JANG1, Min-Su HAN1, Seong-Kwon KIM2, Jong-Sin KIM3
1. Division of Marine System Engineering, Mokpo Maritime University, Mokpo City, Jeonnam 530-729, Korea
2. Media Engineering, Seoul National University of Technology, Seoul 139-743, Korea;
3. Korean Register of Shipping, Tokyo Branch Office, 3rd F, Sanko BLDG’, 4-11,
Nohonbashi Kodenma-cho, Cho-ku, 103-0001, Tokyo, Japan
Received 25 September 2010; accepted 24 December 2010
Abstract:
The corrosions resulting from defects in painting layers frequently occur in Al alloys, so the application of corrosion preventing systems is also very important. Optimum conditions in terms of electrochemistry in relation to solution treatment, quenching and artificial aging treatment were established in order to optimize precipitation strengthening conditions intended to enhance the strength of Al alloys. Slow strain rate tests (SSRT) at various applied potentials were conducted in potential range from -1.8 to -0.5 V. The results show that the maximum tensile strengths, elongations and time-to-fracture are shown to be high values. After precipitation strengthening heat treatment, a tendency appear that time-to-fracture increases as elongation increases. In the potential range from -1.3 V to -0.7 V, the specimens show excellent mechanical properties, and thus this range is considered to be a corrosion prevention range.
Key words:
Al alloy; slow strain rate test; electrochemistry; precipitation strengthening heat treatment; mechanical property;
1 Introduction
FRP materials that have been generally used in leisure boats etc are vulnerable to fires, and hardly reflect radar waves produced by medium/large vessels, resulting in strong possibilities of ship collisions. With the promotion of marine tourism industry in islands regions, there is high possibility of aluminum ships to be built as marine sports ships, fishery guidance ships, maritime police vessels, probes and patrol boats. In particular, since the southwestern coast of Korea has shallow water, the bottom of ship is likely to be bumped against the seabed and thus to be damaged. Furthermore, these vessels are operated at high speeds. Therefore, the development of aluminum alloys with excellent corrosion resistance is very important. Although aluminum is much more corrosion-resistant than steel, it was reported that corrosions resulting from defects in painting layers frequently occur in aluminum boats [1-2]. Therefore, the application of corrosion prevention systems is also very important. Aluminum and its alloys can be strengthened by precipitation strengthening heat treatment. The precipitation strengthening consists of three stages: solution heat treatment, quenching and aging treatment [3]. Aside from the precipitation strengthening, there are mechanical methods including shot peening and friction stir processing. The shot peening is a method to refine surface microstructure by impacting a surface with shot(round steel particles) of high speeds [4-6]. The friction stir processing [7] is an application of friction stir welding [8-10] as a surfacing method that is implemented by rotating a probe placed on the surface of base metal to generate heat, then inserting the probe into the metal. This method is mainly applied for cast products. In the past, heat treatment of aluminum has been mainly approached from the aspect of strength enhancement, and the investigations of heat treatment on electrochemical characteristics are very rare. Optimum conditions in terms of electrochemistry in relation to solution treatment, quenching and artificial aging treatment have been established in order to optimize the precipitation strengthening conditions intended to enhance the strength of aluminum alloys [11]. In this work, the optimum conditions in application of impressed current cathodic protection method were investigated with slow strain rate test (SSRT) for precipitation strengthening heat treated specimens in seawater.
2 Experimental
The 5083F Al alloy(Si 0.09%, Fe 0.289%, Cu 0.018%, Mn 0.589%, Mg 4.449%) was selected as a material among 5000 series alloys. In general, 5000 series are highly strong alloys added with Mg as main additive and they are often used in marine structures or pressure vessels as they have good weldability and corrosion resistance even in seawater environments [12]. The specimens used for electrochemical tests were polished with emery paper up to 1000#, washed with acetone and distilled water and then dried. Polarization experiments were conducted in natural seawater at room temperature using specimens with an exposed area of 1 cm2, a Ag/AgCl electrode as reference electrode and a platinum electrode as counter electrode at a scan rate of 2 mV/s. Anodic polarization was performed from -0.5 V to 3.0 V at an open-circuit potential while cathodic polarization experiments were conducted to -2.0 V at an open-circuit potential. SSRT was conducted with a strain rate of 0.001 mm/min at room temperature with various applied potentials in seawater. Notches(1 mm × 1 mm) were made on parallel areas(16 mm2) in order to induce fracture on parallel parts.
3 Results and discussion
The precipitation strengthening heat treatment progressed in the order of solution treatment, quenching and aging hardening. Solution treatment was carried out at 420 °C with various holding time, and the specimens were water-cooled, thereafter artificial aging was executed at 180 °C for 240 min.
Figure 1 presents the cathodic polarization curves of specimens heat treated at 420 °C for various time, water-cooled, followed by artificial aging at 180 °C for 240 min. In general, the specimens showed a tendency of active dissolution reactions at open-circuit potential, concentration polarization caused by dissolved oxygen reduction reactions (O2 + 2H2O + 4e- → 4OH-) and activation polarization(2H2O + 2e- → H2 + 2OH-) caused by hydrogen gas generation. The current densities of alloys with heat treatment presented low values compared to those in non-heat treatment conditions. It is expected that corrosion resistance will be improved when the impressed current cathodic protection system is applied. In this cathodic polarization curve, the turning points of the concentration polarization induced by dissolved oxygen reduction reactions and the activation polarization are compared with each other, in order to compare the range of cathodic protection potential without effect of hydrogen embrittlement. The optimum solution treatment holding time is determined to be 120 min, showing the best properties.

Fig. 1 Cathodic polarization curves of 5083F Al alloy heat treated at 420 °C for different time
Figure 2 shows the anodic polarization curves of specimens heat treated at 420 °C for various time, water cooled and then artificial aged at 180 °C for 240 min. Since corrosion current densities of heat treated alloys generally presented lower values than those of non-heat treated alloys, it can be said that corrosion resistance was improved by heat treatment. The specimens showed very similar tendencies at above -0.4 V on anodic polarization curve. It is considered that, in that condition, stress corrosion cracking properties of the alloys with heat treatment would not be much different from those of alloys without heat treatment. When the corrosion current densities and corrosion potentials around open circuit potential were compared with each other, the alloy heat treated at this temperature for 120 min showed good characteristics. Therefore, the optimum condition for solution treatment is considered to be conducting heat treatment for 120 min at 410 °C followed by water-cooling and artificial aging treatment for 240 min at 180 °C. In our previous cook, the alloy without heat treatment showed hardness of HV 80.72, thus it could be seen that the hardness values were enhanced by heat treatment [11]. It is considered that heat treatment forms the minutely diffused precipitation matter into the alloy, thereby making the dislocation pass or go around the precipitate particles when external force works. It is considered that strengthening of material is accomplished by the prohibition of dislocation shifting [3].
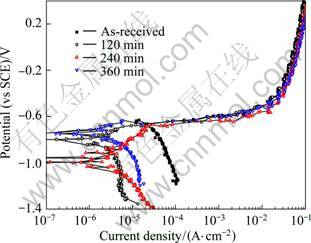
Fig. 2 Anodic polarization curves of 5083F Al alloy with different heat treatment time at 420 °C
Figure 3 represents the relationship between the maximum tensile strength and the applied potentials during SSRT of the alloys with and without precipitation strengthening heat treatment. It was established that the optimum corrosion protection potential of the specimens in non-heat treated state was -1.4 to -0.7 V, where stress corrosion cracking and hydrogen embrittlement would not occur [11]. In this study, potentiostatic SSRTs were conducted in a potential range of -1.9 to -0.4 V, which was wider than the optimum corrosion protection potential range. Based on the results, the maximum tensile strength of the alloys with heat treatment was shown to be higher in the range determined as the optimum protection potential compared with the alloy without heat treatment, and the highest value occurred at -0.7 V. However, the lowest value in corrosion protection range occurred at -0.8 V. The reason for this is considered to be that -0.8 V is close to the natural potential and thus corrosions such as pitting occurs due to the invasion of chloride ions in the seawater environment. In addition, as can be seen through the electrochemical experiments, with increasing potential to the negative direction from the optimum protection corrosion range, hydrogen embrittlement was observed due to the generation of atomic hydrogen or molecular hydrogen. As the potential increased to the noble direction, a tendency of rapid increase in current densities resulting from stress corrosion cracking was shown. Similarly, according to the results of the SSRT, when the tests were conducted in the condition where the specimens were more cathodically or anodically polarized than the optimum corrosion protection range, rapid decrease in the maximum tensile strength value could be observed. The alloys with heat treatment showed better behaviors compared to the alloy without heat treatment in the optimum corrosion protection range.

Fig. 3 Effects of applied potential on maximum tensile strength of alloys during SSRT in sea water
Figure 4 shows the relationship between the yield strength and the applied potential during SSRT in sea water for the precipitation strengthening heat treated specimen. No particular correlations were observed between the yield strength and heat treatment or applied potentials. In the study of the hydrogen embrittlement of high tensile steel in a marine environment, the maximum tensile strength and yield strength did not show any particular correlations with applied potentials, welding methods and post weld heat treatment after welding [13-14]. In addition, like the case of 5456 Al alloy, 5083F Al alloy showed correlations of the maximum tensile strength with the applied potentials while the yield strength did not [15].
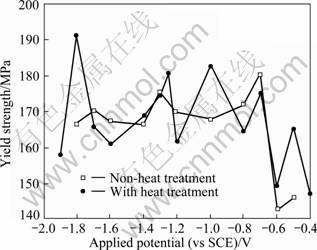
Fig. 4 Effects of applied potential on yield strength of alloys during SSRT in sea water
Figure 5 shows the relationship between elongation and the applied potential during SSRT in seawater for the precipitation strengthening heat treated specimens. In general, elongation is improved by heat treatment. As considered in the previous work on relationship between the maximum tensile strength and the applied potentials, good values were present in the range of the optimum corrosion protection potentials. In SSRT of the specimens polarized further cathodically, elongations decreased with cathodic polarization due to the hydrogen embrittlement caused by atomic hydrogen and molecular hydrogen. When the specimens were polarized more anodically, meanwhile, low elongations were shown due to the increase of dissolution reactions on the metal surfaces. Since, in the precipitation strengthening heat treated condition, anodically polarized potentials showed lower elongations compared to cathodically polarized potentials. It can be seen that the effect of stress corrosion cracking was shown to be larger than that of hydrogen embrittlement.
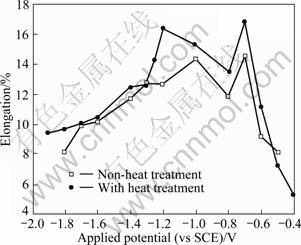
Fig. 5 Effects of applied potential on elongation during SSRT in seawater for precipitation strengthening heat treated specimen
Figure 6 presents the relationship between the time-to-fracture and the applied potential of the specimens with precipitation strengthening heat treatment during SSRT in seawater. It suggests that the heat treatment improved the time-to-fracture. Moreover, the increase in time-to-fracture corresponded to the increasing trend in elongations. This is because elongations would increase with increasing ductility, which makes fracture surface dimple pattern and produces many shear lips, consequently resulting in long time-to-fracture. Especially for the applied potential of -0.7 V, because the elongation and time-to-fracture are long, it is considered that the specimens are under the optimum cathodic protection and are not affected by hydrogen embrittlement and stress corrosion cracking. However, the lowest value of time-to-fracture was shown at -0.8 V in the corrosion protection range. The reason is considered to be that -0.8 V is close to the range of natural potential and thus phenomena such as pitting occurred due to the chloride ion in seawater. When SSRT was carried out for the notched heat-affected zone(HAZ) of high tensile steel in order to assess the characteristics of HAZ, hydrogen embrittlement was observed in the range corresponding to the corrosion protection potential by stress concentration due to the hydrogen attack at the notches. That is, the hydrogen embrittlement was observed at slightly noble potential in notched specimens rather than outside of the notched area [13-14].
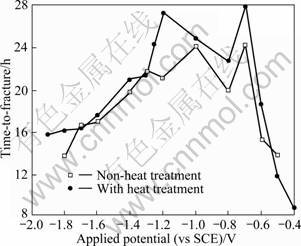
Fig. 6 Effects of applied potential on time-to-fracture during SSRT in seawater for precipitation strengthening heat treated specimen
Figure 7 depicts the fractured specimens of 5083F Al alloy with precipitation strengthening heat treatment when SSRT was conducted in air and at various applied potentials. When the experiments were conducted in air without any effects of seawater, neither the effect of hydrogen embrittlement nor the stress corrosion cracking was observed. The specimens were fractured, showing smooth and clean surface. At the potentials of -0.5 V and -0.6 V, the pitting corrosion occurred simultaneously due to dissolution reactions, which were observed mostly in the center of the parallel area. More dissolution reactions occurred at -0.5 V than at -0.6 V. This is considered to be due to the dissolution reactions progressed under higher current densities in the anodic polarization curve. At the corrosion protection potential range from -1.3 V to -0.7 V, the specimens were fractured with smooth fracture surface. Although the potential of -1.4 V corresponds to corrosion protection potential from electrochemical view, the mechanical properties were decreased based on the results of the SSRT. This is considered to be due to the effect of hydrogen generation on the surfaces of the specimens. In contrast, in the range of -1.8 V to -1.6 V, the formation of white coating on the specimen surface can be seen during corrosion protection in progress. If the cathodic protection is applied in seawater, electrodeposits such as Mg(OH)2 and CaCO3 are precipitated on the surface of the cathode. Although these films consisting of CaCO3 and Mg(OH)2 have corrosion resistant characteristics, it can be seen that thick film formed at -1.7 V to -1.6 V while thin film formed at -1.8 V. This is because more hydrogen gas generation obstructs core creation of electrodeposits. These electrodeposits showed inferior mechanical and electrochemical properties, due to the insufficient time to form compact and thick electrodeposit coating. The incompact electrodeposit coating was formed by hydrogen gas generation. The crevice corrosion by micro galvanic cell formation between the electrodeposit coating and the base metal occurred. The precipitation strengthening heat treatment improved concentration polarization resulting from dissolved oxygen reduction reactions. Therefore, the heat treated specimen would be affected less by hydrogen embrittlement caused by hydrogen gas generation, and consequently the mechanical properties were promoted.

Fig. 7 Photographs of fractured specimen after SSRT for precipitation strengthening heat treated specimens in air and at various applied potentials
Figure 8 presents the fractographs of aluminum alloy 5083F specimens after SSRT in various applied potentials. In air, dimple pattern fracture surface which is typical shape of ductile fracture was generally observed. More dimples and shear lips were observed compared to those of the non-heat treated specimen [11]. At the applied potentials of -0.5 V and -0.6 V, since these potentials correspond to stress corrosion cracking range, the similar fracture surface was observed as that in air while more semi-cleavage fracture was observed. At the applied potential of -0.7 V, where the specimen shows the most excellent mechanical properties, much more dimples and shear lips were observed compared to those at other potentials, suggesting that the elongation was the highest. At the potential of -0.8 V, both dimple fracture and semi-cleavage fracture were observed. In the corrosion potential range from -1.3 V to -1.0 V, dimple fracture surface and the shear lips were generally observed. Besides, at the potentials of -1.8 V to -1.6 V, the effect of hydrogen embrittlement was observed and dimple pattern of ductile fracture surface was very rarely observed. This suggests the short elongation and time-to-fracture. In general, it can be seen that precipitation strengthening heat treatment increased the ductility of specimens. And thus elongation and time-to-fracture were increased.
4 Conclusions
As for the cathodic polarization of precipitation strengthening heat treated 5083F aluminum alloy, a tendency of concentration polarization and activation polarization was observed caused by the reduction reaction of dissolved oxygen and the hydrogen generation, respectively. SSRT conducted in a potential range of -1.8 V to -0.5 V showed high values of the maximum tensile strength, elongation and time-to-fracture. After precipitation strengthening heat treatment, time-to-fracture appeared to be increased with elongation increasing. This is because the ductility increased with increasing elongation. This results many dimple patterns and shear lips of fracture surface. In the applied potentials range of -0.5 V and -0.6 V, stress corrosion cracking occurred. In the range of -1.3 V to -0.7 V, the specimens showed excellent mechanical properties. Since the patterns of semi-cleavage fracture were observed apparently in the potential of -1.8 V to -1.3 V, it is considered that hydrogen embrittlement affected specimens.
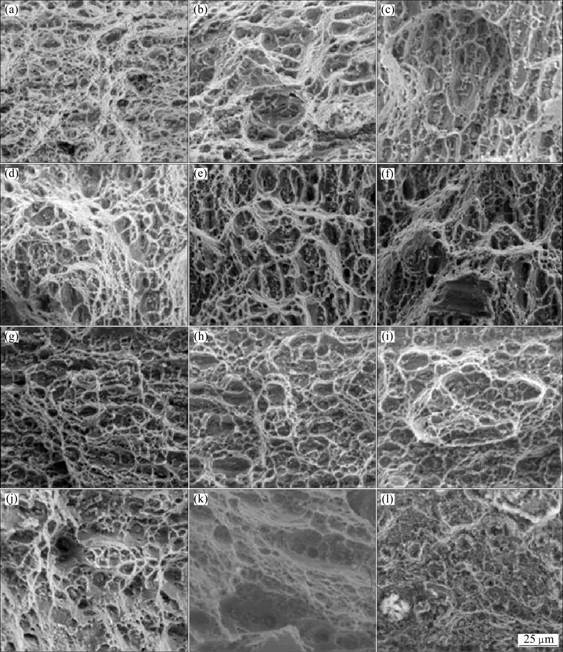
Fig. 8 Fractographs after SSRT for precipitation strengthening heat treated specimens at various applied potentials: (a) In air; (b) -0.5 V; (c) -0.6 V; (d) -0.7 V; (e) -0.8 V; (f) -1.0 V; (g) -1.2 V; (h) -1.25 V; (i) -1.3 V; (j) -1.6 V; (k) -1.7 V; (l) -1.8 V
References
[1] KIM S J, KO J Y. Investigation on optimum protection potential of high-strength Al alloy(5456-H116) for application in ships [J]. Journal of the Korean Soc of Marine Eng, 2006, 30(1): 157-169.
[2] KIM S J. The material and welding technology for Al ship [J]. Journal of the Korean Soc of Marine Eng, 2006, 30(5): 540-551.
[3] KIM C H, KIM S J, KIM H S. Principles of materials science and engineering [M]. Hui Joong Dang, Korea, 1991.
[4] SOYAMA H, PARK J D, SAKA M. Use of cavitating jet for introducing compressive residual stress [J]. Journal of Manufacturing Sci and Eng Trans, ASME, 2000, 122(1): 83-89.
[5] SOYAMA H, KUSAKA T, SAKA M. Peening by the use of cavitation impacts for the improvement of fatigue strength [J]. Journal of Mater Sci Letter, 2001, 20(13): 1263-1266.
[6] SOYAMA H, YAMADA N. Relieving micro-strain by introducing macro-strain in a polycrystalline metal surface by cavitation shotless peening [J]. Materials letters, 2008, 62(20): 3564-3566.
[7] PARK J C, KIM S J. Evaluation of mechanical characteristic and investigation on optimum condition in friction stir processing for 5456-H116 Al alloy [J]. Journal of the Korean Institute of Surface Engineering, 2009, 42(1): 13-20.
[8] SU J W, NELSON T W, STERLING C J. Microstructure evolution during FSW/FSP of high strength aluminum alloys [J]. Materials Science and Engineering A, 2005, 405(1-2): 277-286.
[9] HAN M S, LEE S J, PARK J C, KO S C, WOO Y B, KIM S J. Optimum conditions by mechanical characteristics evaluation in friction stir welding for 5083-O Al alloy [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(s1): s17-s22.
[10] KIM S J, JANG S K. Evaluation of electrochemical characteristic and investigation on optimum condition in friction stir welding for 6061-T6 Al alloy [J]. Journal of the Korean Institute of Surface Engineering, 2008, 41(6): 341-350.
[11] KIM S J, JANG S K. Effects of solution heat treatment on corrosion resistance of 5083F Al alloy [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 887-891.
[12] Korean Register of Shipping. Rules for the classification of steel ships (Part 2): Materials and welding [M]. Korea: Korean Register of Shipping Publications, 2004.
[13] KIM S J, JANG S K, KIM J I. Electrochemical study of hydrogen embrittlement and optimum cathodic protection potential of welded high strength steel [J]. Metals and Mater Inter, 2005, 11(1): 63-69.
[14] KIM S J, MOON V. The relationship between corrosion protection and hydrogen embrittlement properties of HAZ in flux cored arc welding [J]. Metals and Mater Inter, 2002, 8(4): 387-393.
[15] KIM S J, KO J Y, HAN M S. Evaluation of the characteristics using slow strain rate tests of 5456 Al-Mg alloy for ship construction [J]. The Korean Journal of Chemi Engin, 2006, 23(6): 1028-1033.
固溶强化热处理对Al-Mg合金腐蚀性能的影响
Seong-Jong KIM1, Seok-Ki JANG1, Min-Su HAN1, Seong-Kwon KIM2, Jong-Sin KIM3
1. Division of Marine System Engineering, Mokpo Maritime University, Mokpo City, Jeonnam 530-729, Korea
2. Media Engineering, Seoul National University of Technology, Seoul 139-743, Korea;
3. Korean Register of Shipping, Tokyo Branch Office, 3rd F, Sanko BLDG’, 4-11,
Nohonbashi Kodenma-cho, Cho-ku, 103-0001, Tokyo, Japan
摘 要: Al合金表层的缺陷常常会导致腐蚀发生。因此,采取保护措施以提高铝合金的抗腐蚀性能是非常重要的。从电化学的角度出发,对固溶、淬火和时效处理工艺进行优化,以提高铝合金的强度。在不同电位(-05~ -1.8 V)下进行慢应变速率拉伸实验。结果表明:与未经强化处理的铝合金相比,经固溶强化热处理的铝合金表现出高的最大拉伸强度、伸长率和断裂时间。由于经固溶强化处理的铝合金的伸长率增加,其拉伸断裂时间也随之增加。在电位-1.3~-0.7 V范围内,样品具有优异的力学性能,因此,该电位范围被认为是该合金的腐蚀保护范围。
关键词:铝合金;慢应变速率拉伸实验;电化学;固溶强化;热处理;力学性能
(Edited by YUAN Sai-qian)
Corresponding author: Seong-Jong KIM; Tel: +82-61-240-7226; E-mail: ksj@mmu.ac.kr
DOI: 10.1016/S1003-6326(11)60845-5


