Trans. Nonferrous Met. Soc. China 25(2015) 137-145
Effects of Fe2O3 content on microstructure and mechanical properties of CaO-Al2O3-SiO2 system
Xiang-zhong REN, Wei ZHANG, Yong ZHANG, Pei-xin ZHANG, Jian-hong LIU
School of Chemistry and Chemical Engineering, Shenzhen University, Shenzhen 518060, China
Received 28 February 2014; accepted 18 June 2014
Abstract:
The effects of Fe2O3 content on the microstructure and mechanical properties of the CaO-Al2O3-SiO2 system were investigated by differential thermal analysis (DTA), X-ray diffraction (XRD), scanning electron microscopy (SEM), electron spin resonance (ESR), and  spectroscopy. The results show that the addition of Fe2O3 does not affect the main crystalline phase in the prepared glasses, but it reduces the crystallisation peak temperature, increases the crystallisation activation energy, and reduces the crystal granularity. The ESR results indicate that Fe2O3 can promote crystallization, as it leads to the phase separation of the CaO-Al2O3-SiO2 system due to axial distortion. Moreover, Fe2O3 alters the network structure of the CaO-Al2O3-SiO2 system, allowing Fe3+ to enter octahedral sites that exhibit higher symmetry than tetrahedral sites. All of these factors are favourable to increasing the bending strength. The
spectroscopy. The results show that the addition of Fe2O3 does not affect the main crystalline phase in the prepared glasses, but it reduces the crystallisation peak temperature, increases the crystallisation activation energy, and reduces the crystal granularity. The ESR results indicate that Fe2O3 can promote crystallization, as it leads to the phase separation of the CaO-Al2O3-SiO2 system due to axial distortion. Moreover, Fe2O3 alters the network structure of the CaO-Al2O3-SiO2 system, allowing Fe3+ to enter octahedral sites that exhibit higher symmetry than tetrahedral sites. All of these factors are favourable to increasing the bending strength. The  results reveal that there are two types of coordination for both Fe3+ and Fe2+ and the bending strength of the CaO-Al2O3-SiO2 system increases with the amount of six-coordinate Fe3+. The increasing interaction between Fe3+ and Fe2+ can also enhance the bending strength of the CaO-Al2O3-SiO2 system. The microhardness of the CaO-Al2O3- SiO2 system was determined to be HV 896.9 and the bending strength to be 217 MPa under the heat treatment conditions of nucleation temperature of 700 °C and nucleation time of 2 h, crystallization temperature of 910 °C and crystallization time of 3 h.
results reveal that there are two types of coordination for both Fe3+ and Fe2+ and the bending strength of the CaO-Al2O3-SiO2 system increases with the amount of six-coordinate Fe3+. The increasing interaction between Fe3+ and Fe2+ can also enhance the bending strength of the CaO-Al2O3-SiO2 system. The microhardness of the CaO-Al2O3- SiO2 system was determined to be HV 896.9 and the bending strength to be 217 MPa under the heat treatment conditions of nucleation temperature of 700 °C and nucleation time of 2 h, crystallization temperature of 910 °C and crystallization time of 3 h.
Key words:
glass-ceramics; CaO-Al2O3-SiO2 system; Fe2O3; electron paramagnetic resonance; ;spectroscopy; mechanical properties;
1 Introduction
With the rapid development of industrialization processes worldwide and the resultant release and accumulation of slag and tailings, it has been extraordinarily difficult to dispose this waste due to the toxic elements (Pb,Cd and Cu) therein. Therefore, this waste has caused very serious environmental and ecological problems on a global scale. Worldwide, approximately 1×109 t of industrial solid waste is generated each year and 6.7×109 t of industrial waste in total, which is rarely utilized. Slag of glass-ceramics is not only an economically viable but an effective way to deal with this waste. As most slag belongs to the silicate system, and the composition of slag is similar to that of glass-ceramics, slag can easily be formed into glass-ceramics with the addition of a small amount of chemical raw materials. In the glass-ceramics preparation process, the trace toxic elements are completely destroyed and the heavy metals are completely dissolved due to the high melt temperature and heat treatment temperature [1].
Iron oxide comprises a large portion of industrial solid waste. Therefore, it is quite important to study the role of iron oxide in the glass-ceramics preparation process. But it is too hard to study this process with the slag and tailings as materials as they contain lots of elements which would greatly affect the results. Therefore, scholars usually use analytical-grade reagents instead of slag and tailings to study this topic. Many scholars have performed thorough studies of this process. WANG [1] showed that, in the MgO-Al2O3-SiO2 system with cordierite as the main crystalline phase, the iron oxide impurity content had a significant effect on the crystallisation. WANG et al [2] observed that iron oxide could reduce the melting, nucleation, and crystallisation temperatures of the CaO-Al2O3-SiO2-Fe2O3 system. FABREGA et al [3], DANTAS et al [4], and STEFAN et al [5] studied the paramagnetic resonance signal caused by Fe3+ in glass systems. JOHRI et al [6] observed that Fe3+ and Fe2+ coexisted in the 35BaO-40B2O3-25Fe2O3 system after crystallization and exhibited different coordinations, as demonstrated by  spectroscopy. However, all of these studies were purely theoretical and did not consider the mechanical properties of the materials.
spectroscopy. However, all of these studies were purely theoretical and did not consider the mechanical properties of the materials.
In this work, CaO-Al2O3-SiO2 system glass- ceramics with different Fe2O3 contents were synthesized with anorthite as the main crystalline phase. The effects of Fe2O3 content on the microstructure of these glasses were analyzed by DTA, XRD and SEM. Furthermore, the effects of iron valence and content on the microstructure were studied deeply with the help of electron spin resonance (ESR) and  spectroscopy. The changes in mechanical properties caused by the addition of Fe2O3 were evaluated, which may provide powerful theoretical guidance in improving the mechanical properties of slag glass-ceramics.
spectroscopy. The changes in mechanical properties caused by the addition of Fe2O3 were evaluated, which may provide powerful theoretical guidance in improving the mechanical properties of slag glass-ceramics.
2 Experimental
2.1 Preparation of glass-ceramics
The starting materials were analytical-grade CaO, A12O3, SiO2, and Fe2O3 reagents. The mass ratio of CaO to A12O3 and to SiO2 of the synthesized glass-ceramics was 18:30:52, and 4.0% TiO2 (mass fraction) and 4.0% Na2CO3 (mass fraction) were added, thus producing materials with a composition similar to that of fly ash. The added Fe2O3 contents were 0, 2%, 4%, 6%, 8%, and 10% (mass fraction), respectively. After thoroughly mixing, the raw materials were transferred to a 200 mL alumina crucible and melted at 1550 °C for 5 h in an electric furnace in air at a heating rate of 5-10 °C/min. The melts were cast into preheated (500 °C) stainless steel moulds, placed into a preheated (650 °C) furnace for 2 h, and then allowed to cool to room temperature. After lots of experimental data, best heat-treatment conditions were found, the base glass, after being cut to the required dimensions, was fired at 700 °C for 2 h and then heated to 910 °C for 3 h at a heating rate of 5 °C/min. Finally, the samples were allowed to cool to room temperature.
2.2 Characterization
The glass transition temperature and crystallization temperature were obtained from DTA analysis (STA449C, Germany) conducted at a heating rate of 10 °C/min in air. The reference material was γ-Al2O3 powder. The microstructure of the glass-ceramics was observed by SEM (S-570, Japan). The samples were chemically etched for 60 s in 10% HF (mass fraction) and then washed with water. The crystal structure was detected by XRD (D8-ADVANCE, Germany) with Cu Kα radiation at 40 kV and 30 mA over a 2θ range of 10° to 60° at a scanning speed of 2 (°)/min. The microhardness of the samples with dimensions of approximately 10 mm×10 mm×2 mm was tested by a Vickers tester (HXD-1000, China). The bending strength of the samples with dimensions of 5 mm×5 mm×50 mm was tested by a universal electronic tensile machine (CMT4304, China). The samples used to test mechanical properties were heat-treated at the nuclearation temperature of 700 °C and nucleation time of 2 h, crystallization temperature of 910 °C and crystallization time of 3 h. ESR measurements were performed on an electron paramagnetic resonance spectrometer (ER-200D SRC-10/12, Germany) operating at an X-band frequency of 9.05 GHz with 100 kHz magnetic field modulation at room temperature. The samples were in powder form, and the scan width was 800 mT.  experiments were performed on a
experiments were performed on a  tester (unit Mr-260A, Germany) at room temperature with an 57Fe source diffused into a rhodium matrix.
tester (unit Mr-260A, Germany) at room temperature with an 57Fe source diffused into a rhodium matrix.
3 Results and discussion
3.1 DTA and XRD analysis
Figure 1 shows the DTA curves of the base glasses containing different contents of Fe2O3 at a heating rate of 10 °C/min. As shown in Fig. 1, the crystallization peak temperatures (Tp) decreased with increasing Fe2O3 content from 979 °C to 949 °C, which shows that Fe2O3 content greatly affects the crystallization. The crystallization peak temperature of base glasses at different heating rates is shown in Table 1.

Fig. 1 DTA curves of base glass containing different contents of Fe2O3
As is known, when an amorphous phase becomes crystalline, it must overcome a certain energy barrier, called the crystallization activation energy (Ea). This energy is a very important standard for evaluating the material’s capability for crystallization. The following equation is used to calculate Ea [1]:
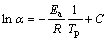 (1)
(1)
where α is the heating rate; R is the universal gas constant; Tp is the crystallization peak temperature in the DTA curve; and C is a constant. The curves of ln α vs 1/Tp can be plotted and Ea values calculated from the slope are shown in Table 2.
Table 1 Crystallization peak temperatures of base glasses at different heating rates
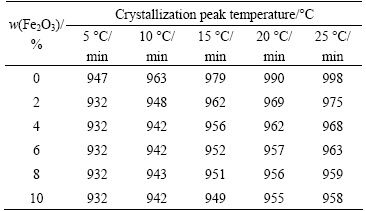
Table 2 Crystallization activation energies of glasses with different Fe2O3 contents

As shown in Tables 1 and 2, the crystallization peak temperature decreased and the crystallisation activation energy of the glasses increased with increasing Fe2O3 content, indicating that these parameters were not directly related [1,7], i.e., the decrease in crystallization peak temperature did not reduce the crystallization activation energy. The reason for this behaviour was that axial distortion tended to become more severe with increasing Fe2O3 content. When the extent of axial distortion reached a certain point, Fe3+ had to find a more stable structure, which led to phase separation and precipitate nucleation, thus promoting crystallization, as shown in Fig. 2. At the same time, as a network former, such as Si4+or Al3+, Fe3+ could form [FeO4] tetrahedrons, mend the net structure, and increase the viscosity of the glass [8], leading to the ions strongly fixed in place. The iron ions caused lattice distortion, increasing the energy of crystal formation and thereby increasing the crystallization activation energy.

Fig. 2 SEM images of glasses without (a) and containing 8% Fe2O3 (b) after nucleation at 700 °C for 2 h
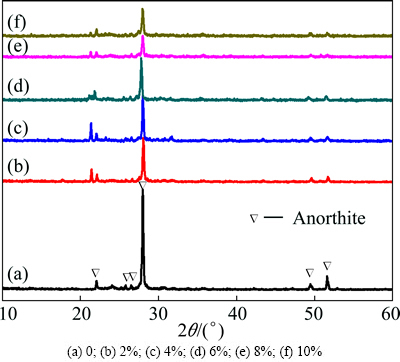
Fig. 3 XRD patterns of glass-ceramics with different Fe2O3 contents
Figure 2 shows SEM images of glasses without Fe2O3 and containing 8% Fe2O3 after the nucleation. As shown in Fig. 2, the sample without Fe2O3 contained few nuclei distributed in the glass phase, whereas the sample with 8% Fe2O3 contained many nuclei distributed homogeneously within the glass phase, indicating that Fe2O3 plays an important role in promoting the crystallization.
Figure 3 shows the XRD patterns of the glass-ceramics with different Fe2O3 contents. As shown in Fig. 3, the Fe2O3 content did not affect the main crystalline phase type. All of the samples contained main phase anorthite (PDF#41-1486). However, with increasing Fe2O3 content, the crystallization peak temperature gradually decreased. As shown in Fig. 4, the crystallization activation energy and full width at half maximum (FWHM) exhibited the same trend with changing Fe2O3 content. Because the higher crystallization activation energies correspond to more difficult migration for the Fe3+ ions, the crystallization was slow and the ions adhered to the nuclei had enough time to adjust their position, indicating the crystal that developed was more ordered. Thus, under the same crystallization conditions, an increase in Fe2O3 content corresponds to a higher extent of crystallization, higher shape regularity, and smaller grains.

Fig. 4 Relationship between FWHM and Ea of glass-ceramics and Fe2O3 content
3.2 Morphology and mechanical properties
Figure 5 shows the SEM images obtained after the heat treatment of the glass-ceramics with different Fe2O3 contents. All samples crystallized well after heat treatment, and spherical grains precipitated. With an increase in Fe2O3 content, the crystallization amount increased but the grains became smaller. This finding is consistent with the data obtained from DTA, XRD, and Ea and FWHM analysis.
Figure 6 shows the flexural strength curve of the glass-ceramics with different Fe2O3 contents. As shown in Fig. 6, with increasing Fe2O3 content, the flexural strength of the system increased almost linearly. As demonstrated in the SEM images in Fig. 5, the granularity of the crystallization gradually decreased with increasing Fe2O3 content. Fine crystal particles can effectively disperse an external force exerted on a point without excessive concentration, abating the stress at the crack tip [9]. Moreover, the crystallization phase can prevent the crack propagation across boundaries, as shown in Fig. 7, thus increasing the flexural strength capacity of the sample.
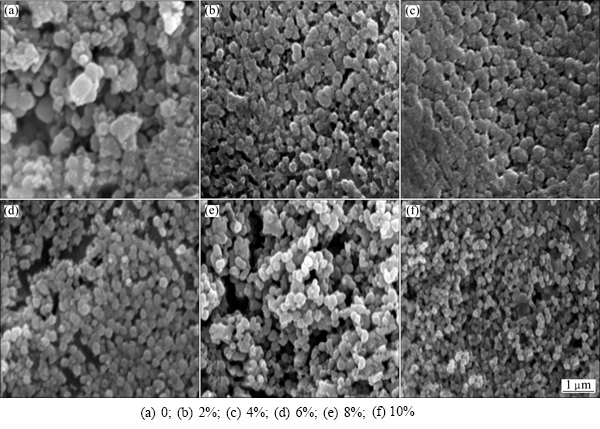
Fig. 5 SEM images of glass-ceramics with different Fe2O3 contents after heat treatment
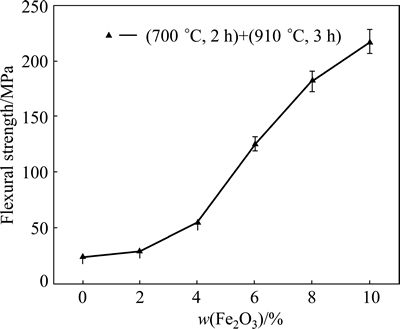
Fig. 6 Flexural strength curve of glass-ceramics with different Fe2O3 contents
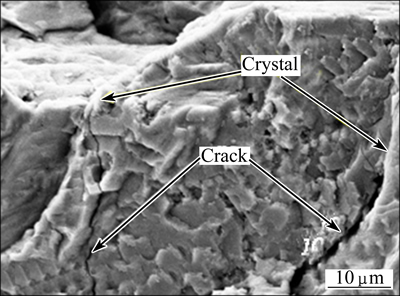
Fig. 7 Crack propagation prevented by crystal structures
Figure 8 shows the SEM images obtained after bending the fracture surface of the glass-ceramics with different Fe2O3 contents. As shown in Fig. 8, when the Fe2O3 contents were 0 and 2%, the fracture surface samples clearly demonstrated cracks. This cracking was caused by different coefficients of thermal expansion between the crystalline and amorphous phases during the annealing process (if cracking was caused by a temperature difference between the surface and the inside of the samples during the annealing process, all samples would exhibit cracking). Because in the heat treatment, the volume expansion was not the same, stress was manifested as varying degrees of shrinkage between the two phases during the cooling process. Furthermore, larger crystal particles experienced more stress. The stress had extremely adverse effects on the mechanical properties of the glass-ceramics. When the Fe2O3 content was 4%, many holes were observed on the fracture surface of the sample. When the Fe2O3 content was 6%, apart from holes, a large number of prominent grains were observed. When the Fe2O3 content was 8% or 10%, a large number of particles were observed without any cracks or holes, which improved the bending strength. The fracture surface gradually became regular, which may be related to the coordination of iron ions. Based on the analysis of the ESR and  spectra, it was found that, with increasing Fe2O3 content, the iron ion coordination transformed from tetrahedral into octahedral, gradually enhancing the symmetry.
spectra, it was found that, with increasing Fe2O3 content, the iron ion coordination transformed from tetrahedral into octahedral, gradually enhancing the symmetry.
Figure 9 shows the microhardness curve of the glass-ceramics with different Fe2O3 contents. The microhardness initially increased with increasing Fe2O3 content when the Fe2O3 content was lower than 6%.
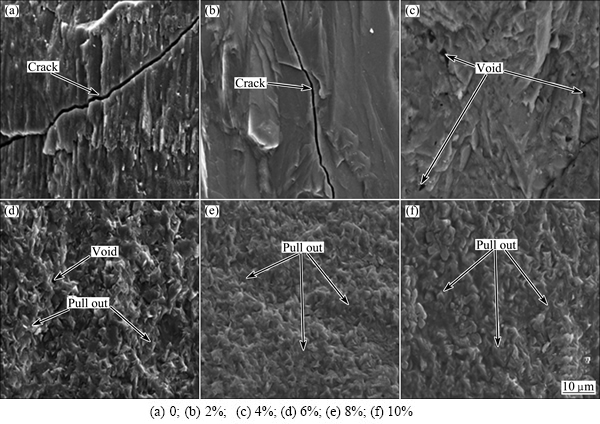
Fig. 8 SEM images of fracture surface of glass-ceramics with different Fe2O3 contents after testing bending strength

Fig. 9 Microhardness curve of glass-ceramics with different Fe2O3 contents
After the Fe2O3 content reached 6%, the microhardness (HV 896.9) decreased with increasing the Fe2O3 content. Hardness is a characteristic that reflects the surface resistance to the compression of another hard object. The size of the hardness indentation is shown in Fig. 10. From Fig. 5, when the Fe2O3 content was 6%, many dense crystals were observed on the sample surface, which would greatly improve the microhardness. “Spherulitic-dendritic” crystals, similarly to plate-like crystals, could promote crack deflection and crack blunting, increase rupture work, and hinder scratching-induced crack propagation through the interface between the crystal phase and glass phase [10]. These properties improved the resistance of the glass-ceramics to external impact.
Figure 10 shows the microhardness indentation curves of the glass-ceramics with different Fe2O3 contents. As shown in Fig. 10, when the Fe2O3 content was 0-4% (Figs. 10(a-c)), higher stress existed in the samples, which created cracks along the indentation edges. Furthermore, with an increase in the Fe2O3 content, the crack length gradually decreased, which was consistent with the bending strength of the fracture surfaces. This behaviour was caused by the stress between different coefficients of thermal expansion of the phases during the annealing process. When the Fe2O3 content was 6%, only a few small holes induced by stress were indicated by the curve, whereas when the Fe2O3 contents were 8% and 10%, respectively, no cracks or holes were indicated by the curves. The changes may be related to the coordination of iron ions, as octahedral coordination is more stable than tetrahedral coordination and the smaller crystal. This finding was a result of the bending strength of the glass-ceramics in terms of applied stress.
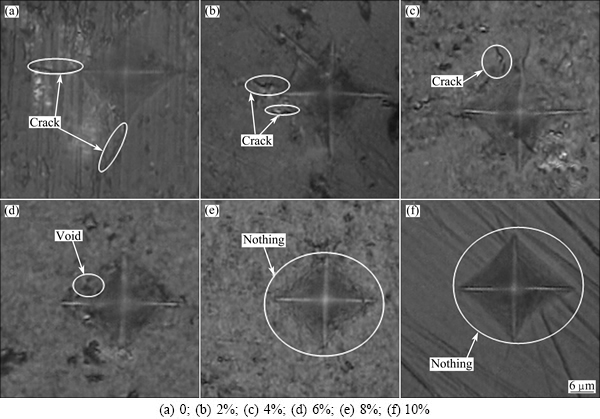
Fig. 10 Microhardness indentation curves of glass-ceramics with different Fe2O3 contents

Fig. 11 Fe3+ ESR absorption spectra of glass-ceramics with different Fe2O3 contents
3.3 ESR and  spectrum analysis
spectrum analysis
Figure 11 shows the Fe3+ ESR absorption spectra of the glass-ceramics with different Fe2O3 contents. The sample without Fe2O3 did not exhibit a Fe3+ absorption peak. The testing parameters were different between the sample without Fe2O3 and the sample containing Fe2O3 (magnetic field scan width: 0-800 mT for the sample containing Fe2O3 and 223-423 mT for the sample with TiO2). Thus, the scan width between 223 and 423 mT was enlarged. The electron configurations Ti atom and Ti4+ ions are 4s23d2 and 3s23p6, respectively. Factor g is an import physical parameter in ESR. It can reflect the interaction between electronic spin motion and orbital motion in paramagnetic molecule for which there is no paramagnetic absorption. The resonance absorption observed at g≈2.598 was due to Ti3+(3d1). Ti4+ was reduced to Ti3+ ions during the melting and heat treatments:
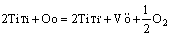 (2)
(2)
The resonance absorption at g≈2.598 is greater than that at factor g of a free electron (ge≈2.0023), indicating that spin coupling existed between the Ti3+ ions and oxygen vacancy defects. Figure 11 shows that the resonance absorption of Ti3+ ions disappeared in the sample with Fe2O3 as Ti3+ ions oxidized to Ti4+ ions:
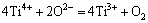 (3)
(3)
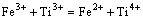 (4)
(4)
Additionally, some of the Fe3+ ions were reduced to Fe2+ ions. The structure of the spectra mainly consisted of absorptions centred at g≈6.0, g≈4.3, and g≈2.1 with their relative intensity being strongly dependent on the Fe2O3 content. The absorptions at g≈6.0 and g≈4.3 were caused by Fe3+ (3d5); the former arose from axially distorted sites, and the latter was characteristic of isolated distorted octahedral or tetrahedral oxygen environments. The resonance absorption at g≈2.1 was due to ion clusters [3-5].
Ion clusters are small regions containing two or more ions coupled together, with their relative intensity being strongly dependent on their composition [11,12]. In the system considered in this study, the intensity of the resonance line the g≈6.0 increased monotonically with increasing Fe2O3 content, indicating that axial distortion tended to become more serious with increasing Fe2O3 content. When the extent of axial distortion reached a certain point, the Fe3+ ion had to find a more stable structure, which led to phase separation and precipitation, thus promoting the crystallization. This mechanism was the basic promoter of crystallization for Fe2O3. The intensity of resonance line at g≈4.3 decreased, and the linewidth increased gradually. This behaviour was observed because the viscosity of the system increased with the addition of Fe2O3. Thus, during the nucleation and crystallization, the ions were strongly fixed and the crystal growth velocity was slow. Therefore, the ions had enough time to adjust the network structure. The crystal became more ordered, and the Fe3+ entered octahedral sites (based on  spectroscopy analysis), thus reducing the absorption intensity. The increase in the linewidth was due to the formation of ion clusters. Two contrary mechanisms affected the linewidth at g≈2.1. Superexchange mechanisms tended to narrow the absorption line, and interaction between Fe2+ and Fe3+ ions tended to broaden the linewidth. Fe2+ did not exhibit ESR absorption, but its interaction with Fe3+ may affect the characteristics of the absorption line [13]. In this system, the increase in the linewidth of the resonance at g≈2.1 with increasing Fe2O3 content indicated the dominance of the broadening mechanisms, and the presence of Fe2+ ions was proven by the
spectroscopy analysis), thus reducing the absorption intensity. The increase in the linewidth was due to the formation of ion clusters. Two contrary mechanisms affected the linewidth at g≈2.1. Superexchange mechanisms tended to narrow the absorption line, and interaction between Fe2+ and Fe3+ ions tended to broaden the linewidth. Fe2+ did not exhibit ESR absorption, but its interaction with Fe3+ may affect the characteristics of the absorption line [13]. In this system, the increase in the linewidth of the resonance at g≈2.1 with increasing Fe2O3 content indicated the dominance of the broadening mechanisms, and the presence of Fe2+ ions was proven by the  spectra (Fig. 12). Considering the bending strength performance curve, the coordination types of Fe3+ had strong effects on the bending strength. The six- coordination led to a higher bending strength, and the increase in the interaction between Fe2+ and Fe3+ was also favourable to the bending strength.
spectra (Fig. 12). Considering the bending strength performance curve, the coordination types of Fe3+ had strong effects on the bending strength. The six- coordination led to a higher bending strength, and the increase in the interaction between Fe2+ and Fe3+ was also favourable to the bending strength.
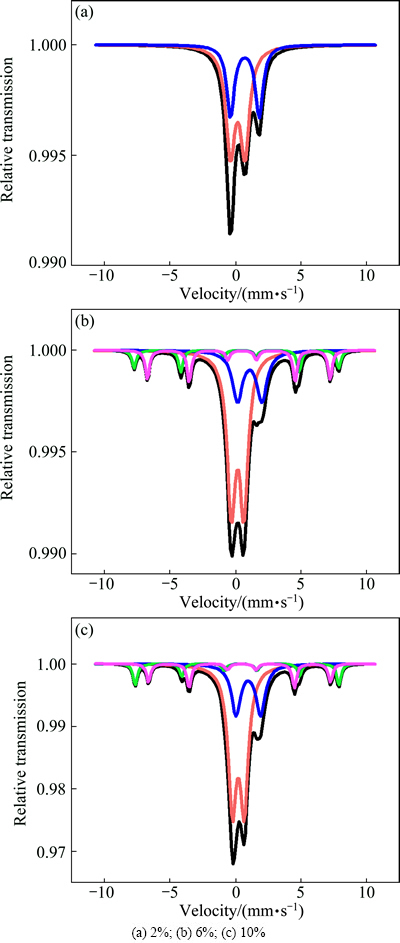
Fig. 12  spectra of glass-ceramics with different Fe2O3 contents
spectra of glass-ceramics with different Fe2O3 contents
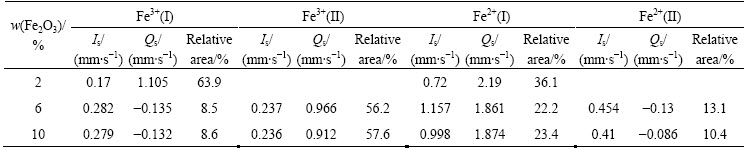
Figure 12 shows the  spectrum of the glass-ceramics with different Fe2O3 contents. The isomer shifts were referred to the standard α-iron. As shown in Fig. 12 and Table 3, the glass-ceramics exhibited quadrupole splitting (Qs) of Fe2+ and Fe3+ ions. The six-line peak signal in the sample containing 2% Fe2O3 was buried in noise because the Fe2O3 content was too low. Fe2+ and Fe3+ ions had the same s-electron number, but the Fe3+ ion volume was smaller, resulting in a larger nuclear vicinity s-electron density. Thus, its isomer shift (Is) was smaller than that of Fe2+, making it easy to distinguish the two ions. As shown in Table 3, the isomer shifts of the magnetic component 0.17-0.279 mm/s and 0.236-0.237 mm/s were characteristics of Fe3+(I) and Fe3+(II), respectively. Certain amount of non-bridging oxygen existed in the [FeO6] octahedron. Compared with the bridge oxygen of the [FeO4] tetrahedral, the Coulomb force was enhanced, thus, the oxygen atom was closer to Fe3+. Consequently, there was a greater overlap of the 2p orbit of the oxygen with the 4s orbit of iron. As a result, an increase in the s-electron density of the iron nuclear site occurred and the Is value decreased [14,15]. Therefore, Fe3+(I) exhibited tetrahedral coordination (coordination number of 4), whereas Fe3+(II) exhibited octahedral coordination (coordination number of 8). Furthermore, as indicated in Table 3, the content of [FeO6] octahedra and the symmetry of Fe3+ increased, causing a decrease in resonance intensity at g≈4.3 (Fig. 11) and an increase in the bending strength (Fig. 6).
spectrum of the glass-ceramics with different Fe2O3 contents. The isomer shifts were referred to the standard α-iron. As shown in Fig. 12 and Table 3, the glass-ceramics exhibited quadrupole splitting (Qs) of Fe2+ and Fe3+ ions. The six-line peak signal in the sample containing 2% Fe2O3 was buried in noise because the Fe2O3 content was too low. Fe2+ and Fe3+ ions had the same s-electron number, but the Fe3+ ion volume was smaller, resulting in a larger nuclear vicinity s-electron density. Thus, its isomer shift (Is) was smaller than that of Fe2+, making it easy to distinguish the two ions. As shown in Table 3, the isomer shifts of the magnetic component 0.17-0.279 mm/s and 0.236-0.237 mm/s were characteristics of Fe3+(I) and Fe3+(II), respectively. Certain amount of non-bridging oxygen existed in the [FeO6] octahedron. Compared with the bridge oxygen of the [FeO4] tetrahedral, the Coulomb force was enhanced, thus, the oxygen atom was closer to Fe3+. Consequently, there was a greater overlap of the 2p orbit of the oxygen with the 4s orbit of iron. As a result, an increase in the s-electron density of the iron nuclear site occurred and the Is value decreased [14,15]. Therefore, Fe3+(I) exhibited tetrahedral coordination (coordination number of 4), whereas Fe3+(II) exhibited octahedral coordination (coordination number of 8). Furthermore, as indicated in Table 3, the content of [FeO6] octahedra and the symmetry of Fe3+ increased, causing a decrease in resonance intensity at g≈4.3 (Fig. 11) and an increase in the bending strength (Fig. 6).
For Fe2+ ions, the isomer shift values of 0.997±0.005 was characteristic of Fe2+ dispersed in the glass. In the ESR curve (Fig. 11), the increase in the linewidth of resonance g≈2.1 stemmed from the interaction between the Fe2+ and Fe3+ ions.
4 Conclusions
1) The crystallization peak temperature decreases, the crystallisation activation energy increases, and the crystal granularity decreases with the addition of Fe2O3 in the CaO-Al2O3-SiO2 system.
2) The ESR results indicate that Fe2O3 can adjust the network structure of the glass-ceramics, allowing Fe3+ to assume an octahedral coordination, which enhances the bending strength of the glass-ceramics.
3) The  spectroscopy results reveal two types of coordinations in both Fe2+and Fe3+ ions, and the bending strength increases with increasingly prevalent Fe3+ six-coordination. Moreover, the interaction between Fe2+ and Fe3+ ions can also enhance the bending strength of the glass-ceramics.
spectroscopy results reveal two types of coordinations in both Fe2+and Fe3+ ions, and the bending strength increases with increasingly prevalent Fe3+ six-coordination. Moreover, the interaction between Fe2+ and Fe3+ ions can also enhance the bending strength of the glass-ceramics.
4) The microhardness is HV 896.9 and the bending strength is 217 MPa for CaO-Al2O3-SiO2 system under heat treatment conditions of (700 °C, 2 h)+(910 °C, 3 h).
Table 3  spectroscopy parameters of iron in glass-ceramics with different Fe2O3 contents
spectroscopy parameters of iron in glass-ceramics with different Fe2O3 contents
References
[1] WANG S M. Effects of Fe on crystallization and properties of a new high infrared radiance glass-ceramics [J]. Environmental Science & Technology, 2010, 44(12): 4816-4820.
[2] WANG Z J, NI W, LI K Q, HUANG X Y, ZHU L P. Crystallization characteristics of iron-rich glass ceramics prepared from nickel slag and blast furnace slag [J]. International Journal of Minerals, Metallurgy and Materials, 2011, 18(4): 455-459.
[3] FABREGA C, ANDREU T, CABOT A, MORANTE J R. Location and catalytic role of iron species in TiO2: Fe photocatalysts: An EPR study [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2010, 211(2-3): 170-175.
[4] DANTAS N O, AYTA W E F, SILVA A C A, CANO N F, RODRIGUEZ A F R, OLIVEIRA A C, GARG V K, MORAIS P C. Magnetic and optical investigation of 40SiO2·30Na2O·1Al2O3·(29-x) B2O3·xFe2O3 glass matrix [J]. Solid State Sciences, 2012, 14(8): 1169-1174.
[5] STEFAN R, PASCUTA P, POPA A, RAITA O, INDREA E, CULEA E. XRD and EPR structural investigation of some zinc borate glasses doped with iron ions [J]. Journal of Physics and Chemistry of Solids, 2012, 73(2): 221-226.
[6] JOHRI U C, SINGRU R M, BAHADUR D.  spectroscopic studies of glass ceramics with hexagonal barium and strontium ferrites [J]. Journal of Materials Science, 1992, 27(22): 6223-6228.
spectroscopic studies of glass ceramics with hexagonal barium and strontium ferrites [J]. Journal of Materials Science, 1992, 27(22): 6223-6228.
[7] PARK Y J, HEO J. Nucleation and crystallization kinetics of glass derived from incinerator fly ash waste [J]. Ceramics International, 2002, 28(6): 669-673.
[8] WANG Z J, NI W, JIA Y, ZHU L P, HUANG X Y. Crystallization behavior of glass ceramics prepared from the mixture of nickel slag, blast furnace slag and quartz sand [J]. Journal of Non-Crystalline Solids, 2010, 356(31-32): 1554-1558.
[9] IKEDA K, KINOSHITA H, KAWAMURA R, YOSHIKAWA A, KOBORI O, HIRATSUKA A. Production of high-strength glass-ceramics from industrial wastes using phase equilibrium diagram of CaO-Al2O3-SiO2 system [J]. Journal of Solid Mechanics and Materials Engineering, 2011, 5(5): 209-221.
[10] ROY S, BASU B. Hardness properties and microscopic investigation of crack-crystal interaction in SiO2-MgO-Al2O3-K2O-B2O3-F glass ceramic system [J]. Journal of Materials Science: Materials in Medicine, 2010, 21(1): 109-122.
[11] RAJENDRA K S, KOTHIYAL G P, SRINIVASAN A. Electron spin resonance and magnetic studies on CaO-SiO2-P2O5-Na2O-Fe2O3 glasses [J]. Journal of Non-Crystalline Solids, 2008, 354(27): 3166-3170.
[12] RAJENDRA K S, KOTHIYAL G P, SRINIVASAN A. Influence of iron ions on the magnetic properties of CaO-SiO2-P2O5-Na2O- Fe2O3 glass-ceramics [J]. Solid State Communications, 2008, 146(1-2): 25-29.
[13] ARAUJO E B D, PAIVA J A C D, ARAUJO M A B D, SOMBRA A S B. Structure and optical properties of nithium iobium-phosphate glasses and glass ceramics [J]. Physica Status Solidi, 1996, 197: 231-240.
[14] CHAUDHURI S P, PATUA S K. Electron paramagnetic resonance and  spectroscopy of transition metal ion doped mullite [J]. Journal of Materials Science, 2000, 35(18): 4735-4741.
spectroscopy of transition metal ion doped mullite [J]. Journal of Materials Science, 2000, 35(18): 4735-4741.
[15] SDIRI N, ELHOUICHET H, AZEZA B, MOKHTAR F. Studies of (90-x)P2O5-xB2O3-10Fe2O3 glasses by  effect and impedance spectroscopy methods [J]. Journal of Non-Crystalline Solids, 2013, 371-372: 22-27.
effect and impedance spectroscopy methods [J]. Journal of Non-Crystalline Solids, 2013, 371-372: 22-27.
Fe2O3含量对CaO-Al2O3-SiO2系微晶玻璃显微组织与力学性能的影响
任祥忠,张 卫,章 勇,张培新,刘剑洪
深圳大学 化学与化工学院,深圳 518060
摘 要:采用差热分析(DTA)、X射线衍射(XRD)、扫描电镜(SEM)、电子顺磁共振(ESR)和 谱等技术研究Fe2O3含量对CaO-Al2O3-SiO2系微晶玻璃显微组织与力学性能的影响。结果表明:Fe2O3的加入不改变CaO-Al2O3-SiO2系微晶玻璃析出的主晶相类型,但使体系的析晶峰温度降低,析晶活化能增加和析出晶体的粒度减小。ESR测试结果表明,Fe2O3的加入会因轴向扭曲造成分相,从而促进析晶;同时,Fe2O3的加入能更好地调整CaO-Al2O3-SiO2系微晶玻璃网络内部结构,使Fe3+进入比四面体对称性更高的八面体配位,有利于抗弯强度的增大。
谱等技术研究Fe2O3含量对CaO-Al2O3-SiO2系微晶玻璃显微组织与力学性能的影响。结果表明:Fe2O3的加入不改变CaO-Al2O3-SiO2系微晶玻璃析出的主晶相类型,但使体系的析晶峰温度降低,析晶活化能增加和析出晶体的粒度减小。ESR测试结果表明,Fe2O3的加入会因轴向扭曲造成分相,从而促进析晶;同时,Fe2O3的加入能更好地调整CaO-Al2O3-SiO2系微晶玻璃网络内部结构,使Fe3+进入比四面体对称性更高的八面体配位,有利于抗弯强度的增大。 测试结果表明,Fe3+和Fe2+在CaO-Al2O3-SiO2系微晶玻璃存在不同的配位,且微晶玻璃的抗弯强度随Fe3+六配位数的增多而增大;同时,Fe2+和Fe3+相互作用的增强也有利于微晶玻璃抗弯强度的增大。在核化温度为700 °C、核化时间为2 h、晶化温度为910 °C和晶化时间为3 h的热处理条件下,样品的显微硬度达到HV 896.9,抗弯强度达到217 MPa。
测试结果表明,Fe3+和Fe2+在CaO-Al2O3-SiO2系微晶玻璃存在不同的配位,且微晶玻璃的抗弯强度随Fe3+六配位数的增多而增大;同时,Fe2+和Fe3+相互作用的增强也有利于微晶玻璃抗弯强度的增大。在核化温度为700 °C、核化时间为2 h、晶化温度为910 °C和晶化时间为3 h的热处理条件下,样品的显微硬度达到HV 896.9,抗弯强度达到217 MPa。
关键词:微晶玻璃;CaO-Al2O3-SiO2系;Fe2O3;电子顺磁共振; 谱;力学性能
谱;力学性能
(Edited by Wei-ping CHEN)
Foundation item: Project (50974090) supported by the National Natural Science Foundation of China; Projects (JCYJ20140418182819155, JCYJ20130329113849606) supported by the Shenzhen Dedicated Funding of Strategic Emerging Industry Development Program, China
Corresponding author: Pei-xin ZHANG; Tel: +86-755-26558134; E-mail: pxzhang2000@163.com
DOI: 10.1016/S1003-6326(15)63588-9
Abstract: The effects of Fe2O3 content on the microstructure and mechanical properties of the CaO-Al2O3-SiO2 system were investigated by differential thermal analysis (DTA), X-ray diffraction (XRD), scanning electron microscopy (SEM), electron spin resonance (ESR), and  spectroscopy. The results show that the addition of Fe2O3 does not affect the main crystalline phase in the prepared glasses, but it reduces the crystallisation peak temperature, increases the crystallisation activation energy, and reduces the crystal granularity. The ESR results indicate that Fe2O3 can promote crystallization, as it leads to the phase separation of the CaO-Al2O3-SiO2 system due to axial distortion. Moreover, Fe2O3 alters the network structure of the CaO-Al2O3-SiO2 system, allowing Fe3+ to enter octahedral sites that exhibit higher symmetry than tetrahedral sites. All of these factors are favourable to increasing the bending strength. The
spectroscopy. The results show that the addition of Fe2O3 does not affect the main crystalline phase in the prepared glasses, but it reduces the crystallisation peak temperature, increases the crystallisation activation energy, and reduces the crystal granularity. The ESR results indicate that Fe2O3 can promote crystallization, as it leads to the phase separation of the CaO-Al2O3-SiO2 system due to axial distortion. Moreover, Fe2O3 alters the network structure of the CaO-Al2O3-SiO2 system, allowing Fe3+ to enter octahedral sites that exhibit higher symmetry than tetrahedral sites. All of these factors are favourable to increasing the bending strength. The  results reveal that there are two types of coordination for both Fe3+ and Fe2+ and the bending strength of the CaO-Al2O3-SiO2 system increases with the amount of six-coordinate Fe3+. The increasing interaction between Fe3+ and Fe2+ can also enhance the bending strength of the CaO-Al2O3-SiO2 system. The microhardness of the CaO-Al2O3- SiO2 system was determined to be HV 896.9 and the bending strength to be 217 MPa under the heat treatment conditions of nucleation temperature of 700 °C and nucleation time of 2 h, crystallization temperature of 910 °C and crystallization time of 3 h.
results reveal that there are two types of coordination for both Fe3+ and Fe2+ and the bending strength of the CaO-Al2O3-SiO2 system increases with the amount of six-coordinate Fe3+. The increasing interaction between Fe3+ and Fe2+ can also enhance the bending strength of the CaO-Al2O3-SiO2 system. The microhardness of the CaO-Al2O3- SiO2 system was determined to be HV 896.9 and the bending strength to be 217 MPa under the heat treatment conditions of nucleation temperature of 700 °C and nucleation time of 2 h, crystallization temperature of 910 °C and crystallization time of 3 h.


