
Microstructure and synthesis mechanism of Al-Ti-C-Sr master alloy
ZHAO Hong-liang(赵红亮), WANG Jun(王 军), SONG Yong(宋 勇), GUAN Shao-kang(关绍康)
School of Materials Science and Engineering, Zhengzhou University, Zhengzhou 450002, China
Received 16 April 2009; accepted 13 July 2009
Abstract:
Al-5Ti-0.5C-8Sr (mass fraction, %) master alloy was prepared using a melt reaction method. The microstructure and synthetic process of the master alloy were investigated by optical microscopy, X-ray diffraction, scanning electron microscopy and X-ray energy-dispersive spectrum. The results show that the master alloy is composed of α(Al), TiAl3, TiC, Al4Sr and Al-Ti-Sr phases. The synthesis mechanisms of the master alloy are as follows: TiAl3 is formed through the reaction between K2TiF6 and Al melt at 850 ℃; when the melt was heated up to 1 200-1 300 ℃, TiC was formed through the reaction: Ti+C(s)=TiC(s); Al4Sr was formed through the binary uniform reaction when Sr was added into the melt; after the following solidification process in the peritectic reaction: L(Al, Sr)+α(TiAl3)→β(Al-Ti-Sr), the enwrapped structure was formed with the outer layer of Al-Ti-Sr phase and the internal layer of TiAl3 phase.
Key words:
Al-Ti-C-Sr alloy; synthesis mechanism; microstructure; Al-Ti-Sr phase;
1 Introduction
Al-Ti-B and Al-Ti-C master alloys are good refiners for Al and its alloys[1-3]. Compared with TiB2 in Al-Ti-B master alloys, TiC particles in Al-Ti-C grain refiner as the heterogeneous nucleation core have smaller aggregation tendency which is not affected by such elements as Zr, Cr, Mn and V. Therefore, more attention has been paid to the preparation, microstructure and performance of Al-Ti-C master alloys[1, 4-5].
Al-Sr alloys are widely used in industrial practice for the modification of Al-Si alloys, in which the eutectic Si is converted from a coarse flake into a fine fibrous morphology[6]. There are many reports on the grain refinement and modification of Al-Si casting alloys by simultaneous addition of grain refiners and modifiers[7-12].
SAGSTAD and BHONDUS[13] synthesized a new Al-Ti-B-Sr master alloy which results in combined grain refinement and modification. But it is reported that B and Sr have mutual poisoning effect because of the formation of SrB6 compound[14]. However, little information is available on the Al-Ti-C-Sr alloy as grain refiner of Al-Si casting alloys.
In previous work, the authors have developed Al-Ti-C-Sr master alloy[15], in which satisfactory grain refining and modifying effects were obtained. In this work, a novel Al-5Ti-0.5C-8Sr master alloy is prepared by a melt reaction method, and the synthetic process and microstructure of the master alloy are investigated in order to improve its refining and modifying effects.
2 Experimental
Al-5Ti-0.5C-8Sr alloy was prepared in the laboratory by the melt reaction method. Commercial pure K2TiF6 (98% purity), graphite powder, commercial pure strontium (99.9% purity), aluminium powder (99% purity) and commercial pure Al (99.7% purity) were used to prepare the Al-Ti-C-Sr master alloy. Fig.1 presents the preparation process of Al-5Ti-0.5C-8Sr alloy. Initially, commercial pure aluminium was melted in medium frequency furnace. The pretreated K2TiF6, graphite powder and aluminium powders were added to the superheated aluminum melt at 800-900 ℃. Then the covering flux was sprinkled to the surface of the melt. After several minutes (the melt was poured partially and the samples were designated as Sample 1 and Sample 2, respectively.), the temperature was increased to 1 200- 1 300 ℃ (the melt was also poured partially and the sample was designated as Sample 3), which was kept for several minutes. Then the temperature was decreased to 800-900 ℃, and pure strontium was added to the melt
after the slag flux was eliminated. After being treated at the temperature of 850 ℃ for 10 min, the melt was poured into a permanent mold (25 mm in diameter and 100 mm in height) and the sample was designated as Sample 4. The metallographic specimens of Al-5Ti-0.5C- 8Sr were polished and etched with an aqueous solution of 0.5% HF. Optical microscopy, scanning electron microscopy, and X-ray energy-dispersive spectrum were used to analyze their microstructures and components, X-ray diffraction was used to identify the phases of the specimen.
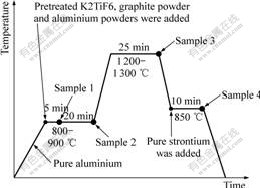
Fig.1 Preparation process of Al-5Ti-0.5C-8Sr alloy
3 Results and discussion
3.1 Microstructure of Al-5Ti-0.5C-8Sr alloy
Fig.2 presents the XRD pattern of Al-5Ti-0.5C-8Sr alloy. It can be seen that the alloy contains α(Al), TiAl3, TiC, Al4Sr and unknown phases. The optical micrograph (Fig.3(a)) of the alloy shows the presence of discrete lath-like, blocky-like, enwrapped-like, particle-like and cluster-like phases in the Al matrix. At higher magnification, it can be seen that the enwrapped-like structure (Fig.3(b)) in the alloy consists of a outer layer for irregular light gray phase and a internal layer for deep gray phase. Fig.4(a) shows the SEM morphology of TiC
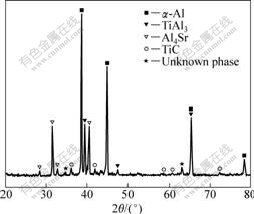
Fig.2 XRD pattern of Al-5Ti-0.5C-8Sr alloy

Fig.3 Optical micrographs of Al-5Ti-0.5C-8Sr alloy
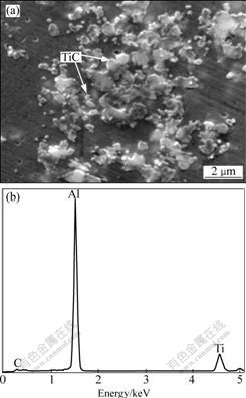
Fig.4 SEM image (a) and EDS spectrum (b) of TiC in Al-5Ti-0.5C-8Sr alloy
at higher magnification. It is seen that some of the near-spherical TiC particles with diameters of 0.5-1.5 μm are dispersed in the Al matrix, and some TiC particles connect with each other to form cluster-like structures or agglomerate together. The EDS spectrum of the fine TiC particles (Fig.4(b)) shows the presence of Al, Ti and C. Due to the fineness of the TiC particles, the Al peak in the spectrum is possibly the contribution of the matrix Al.
Fig.5(a) shows the SEM photograph of the phases (Points A, B and C as indicated by the arrows) in Al-5Ti-0.5C-8Sr alloy. EDS analyses were performed on these phases in Figs.5(b)-(d), respectively. The results indicate that the discrete lath-shaped phase (Point A) is Al4Sr.
From Fig.5(c), the mole ratio of Al to Ti of the internal layer phase (Point B) of enwrapped structure is close to 3:1. Hence, the internal layer phase is identified as TiAl3. Fig.5(d) shows that the outer layer phase (Point C) of enwrapped structure contains Al,Ti and Sr. Moreover, the mole ratio of Ti to Sr is close to 2?1. Fig.6(a) shows the SEM photograph of blocky phase in Al-5Ti-0.5C-8Sr alloy. The EDS patterns of Points D and E indicated by the arrows in Fig.6(a) are shown in Figs.6(b) and (c). The results indicate the blocky-like phase also contains Al, Ti and Sr, and the mole ratio of Ti to Sr is close to 2?1. According to the EDS patterns in Figs.5 and 6, it is presumed that the outer layer of enwrapped structure and the blocky phase are the same phase.
The α(Al) matrix (point E) was analyzed by EDS as shown in Fig.6(c). It can be seen that the α(Al) contains very few Ti atoms and Sr atoms. According to the phase diagrams of Al-Ti and Al-Sr binary alloys, the solubility of Sr and Ti atoms in the α(Al) is very low. In this study, the outer layer of enwrapped structure and the blocky-like phase’s content of Ti and Sr is very high, so it is suggested that α(Al) solid solution phase was not formed, it should be a ternary intermetallic compound containing Al, Ti and Sr. However, the intermetallic compound is not shown in Al-Ti-Sr ternary phase diagram at present. Therefore, it can be presumed that the intermetallic compound may be the unknown phase in Fig.2, and it is taken as Al-Ti-Sr phase.
3.2 Microstructures of Al-5Ti-0.5C-8Sr alloy prepared at different stages
Fig.7 shows the microstructures of Al-5Ti-0.5C-8Sr alloy prepared at different stages. From Fig.7(a), it is found that a lot of TiAl3 particles were fine blocky or short stick-like and dispersed in the α(Al) matrix. When the melt was held for 25 min, the fine blocky TiAl3 particles were replaced by the big lath-like TiAl3 particles (Fig.7(b)). When the melt was heated up to 1 200-1 300 ℃ and hold for 25 min (Fig.7(c), the microstructure of Sample 3), the big lath-like TiAl3
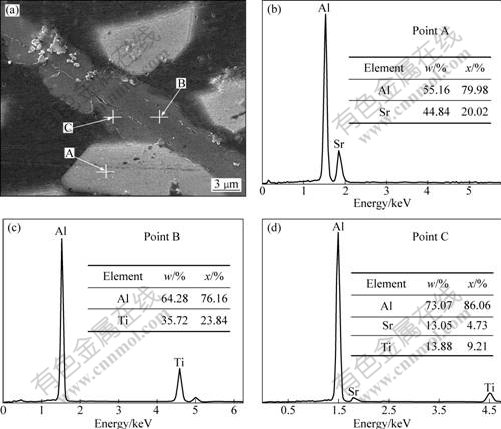
Fig.5 SEM image of Al-5Ti-0.5C-8Sr alloy (a), EDS spectra of point A (b), point B (c) and point C (d), respectively

Fig.6 SEM image of blocky phase in Al-5Ti-0.5C-8Sr alloy (a), EDS spectrum of Point D (b) and EDS spectrum of Point E (c)
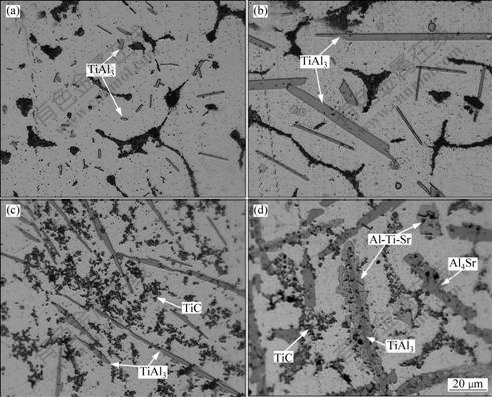
Fig.7 Optical micrographs of Sample 1 (a), Sample 2 (b), Sample 3 (c) and Sample 4 (d)
particles were replaced by the irregular needle-like TiAl3 particles, a mass of dispersed particles with small size were distributed in α(Al) matrix. Some of the particles have a little agglomeration. According to the analyses in Section 3.1, it is found that there were discrete lath-like Al4Sr, particle-like and cluster-like TiC in Sample 4(Fig.7(d)), some blocky Al-Ti-Sr and enwrapped structure composed of outer layer of Al-Ti-Sr phase and internal layer of TiAl3 phase.
3.3 Formation mechanism of Al-5Ti- 0.5C-8Sr alloy
The forming process of TiAl3 and TiC are explained as follows: the appearance of little blocky and short stick-like TiAl3 (Fig.6(a)) is attributed to the following reaction[16]:
K2TiF6+Al→TiAl3+KA1F4+K3AlF6 (1)
which happened at 850 ℃ at the interface between K2TiF6 which has a low melting point, and liquid aluminum. The little blocky TiAl3 grows up to big lath-like (Fig.7(b)), which is attributed to the long holding time. When the temperature of melt is heated up to 1 200-1 300 ℃, the released Ti atoms form K2TiF6 spread to the high-temperature activated C interface, and TiC (Fig.7(b)) is formed through the reaction[17]:
Ti+C(s)=TiC(s) (2)
Finally, when the alloy melt is solidified, the fine acicular TiAl3 (Fig.7(c)) phase precipitates from the melt. At higher pouring temperature, the solubility of Ti in aluminum melt is high and the melt becomes uniform. Restricted by the growth condition along [001] orientation, the growth rate of TiAl3 crystal along [001] was low. Its growth depends on the dominant process of Ti diffusing to the crystal edge in the melt, hence the trend of preferential growth increased obviously and TiAl3 exhibits a acicular-like structure[4, 18].
Moreover, it can be seen that some of TiC particles agglomerate together in Fig. 7(d), which is attributed to the long holding time at high temperature. The fine dispersed TiC particles with higher surface energy at high temperature are not stable in the Al matrix, the fine dispersed TiC is prone to agglomerate to keep a more stable state in the final solidification process, which complies with the minimum energy principle.
According to Al-Sr and Al-Ti alloy phase diagrams, the forming process of Al4Sr and Al-Ti-Sr phases can be construed as follows: primary Al4Sr is formed in the melt through uniform reaction after pure Sr was added at 850 oC. After the following solidification process, the excessive Al atoms and Sr atoms tend to deposit onto the surface of the primary TiAl3 and grow up via peritectic reaction continuously. Meanwhile, Ti atoms from the enwrapped TiAl3 successively spread to the outer layer, and then form the Al-Ti-Sr phase. The possible peritectic reaction is
L(Al, Sr)+α(TiAl3)→β(Al-Ti-Sr) (3)
Actually, peritectic reaction cannot carry on completely in a real process, because the temperature of melt in the solidification process dropped rapidly, that the internal layer of the enwrapped structure is the remaining TiAl3 and the outer layer formed the new Al-Ti-Sr phase ( Fig.7(d)).
![]() In order to further confirm this assumption, Al-5Ti-0.5C-8Sr alloy was annealed at 620 ℃ for 18 h, the optical micrograph is shown in Fig.8. It can be seen that the internal layer acicular of the enwrapped structure is almost exhausted, the main structure is Al-Ti-Sr phase. It can be explained that the further spread of the soluble atoms in the enwrapped structure get through the layers to react for the long holding time. Finally, the peritectic reaction finished thoroughly: L(Al, Sr)+α(TiAl3)→ β(Al-Ti-Sr).
In order to further confirm this assumption, Al-5Ti-0.5C-8Sr alloy was annealed at 620 ℃ for 18 h, the optical micrograph is shown in Fig.8. It can be seen that the internal layer acicular of the enwrapped structure is almost exhausted, the main structure is Al-Ti-Sr phase. It can be explained that the further spread of the soluble atoms in the enwrapped structure get through the layers to react for the long holding time. Finally, the peritectic reaction finished thoroughly: L(Al, Sr)+α(TiAl3)→ β(Al-Ti-Sr).
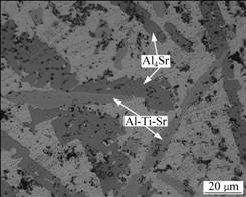
Fig.8 Optical micrograph of Al-Ti-Sr in Al-5Ti-0.5C-8Sr alloy after annealing
4 Conclusions
1) Al-5Ti-0.25C-8Sr master alloy was prepared by a melt reaction method. The microstructure of the alloy was composed of α(Al), non-continuous lath-like Al4Sr, particle-like or cluster-like TiC, enwrapped-like TiAl3, blocky-like and rim Al-Ti-Sr.
2) TiAl3 was formed through the reaction between K2TiF6 and Al melt at 850 ℃; when the melt was heated up to 1 200-1 300 ℃, TiC was formed through the reaction: Ti+C(s)→TiC(s); when Sr was added into the melt at 850 ℃, Al4Sr was formed through the binary uniform reaction.
3) After the following solidification process in the peritectic reaction: L(Al, Sr)+α(TiAl3)→β(Al-Ti-Sr), the enwrapped structure was formed with the outer layer of Al-Ti-Sr phase and the internal layer of TiAl3 phase.
References
[1] KUMAR G S, MURTY B S, CHAKRABORTY M. Development of Al-Ti-C grain refiners and study of their grain refining efficiency on Al and Al-7Si alloy [J]. Journal of Alloys and Compounds, 2005, 396: 143-150.
[2] Yücel B. Grain refining efficiency of Al-Ti-C alloys [J]. Journal of Alloys and Compounds, 2006, 422: 128-131.
[3] KUMAR G S, MURTY B S, CHAKRABORTY M. Grain refinement response of LM25 alloy towards Al-Ti-C and Al-Ti-B grain refiners [J]. Journal of Alloys and Compounds, 2009, 472: 112-120.
[4] LI Y L, FENG H K, CAO F R, CHEN Y B, GONG L Y. Effect of high density ultrasonic on the microstructure and refining property of Al–5Ti–0.25C grain refiner alloy [J]. Materials Science and Engineering A, 2008, 487: 518-523.
[5] LIU Xiang-fa, WANG Zhen-qing, ZHANG Zuo-gui, BIAN Xiu-fang. The relationship between microstructures and refining performances of Al–Ti–C master alloys [J]. Materials Science and Engineering A, 2002, 332: 70-74.
[6] CHANG J Y, KO H S. Twin probability of eutectic Si in rare earth modified Al-7wt% Si alloy [J]. Mater Sci Lett, 2000, 19: 197-199.
[7] KORI S A, MURTY B S, CHAKRABORTY M. Development of an efficient grain refiner for Al-7Si alloy [J]. Materials Science and Engineering A, 2000, 280: 58-61.
[8] LI J G, ZHANG B Q, WANG L, YANG W Y, MA H T. Combined effect and its mechanism of Al–3wt.%Ti–4wt.%B and Al–10wt.%Sr master alloy on microstructures of Al–Si–Cu alloy [J]. Materials Science and Engineering A, 2002, 328: 169-176.
[9] LIAO Heng-cheng, SUN Guo-xiong. Mutual poisoning effect between Sr and B in Al–Si casting alloys [J]. Scripta Mater, 2003, 48: 1035-1039.
[10] RAO A K, MURTY B S, CHAKRABORTY M. Improvement in tensile strength and load bearing capacity during dry wear of Al–7Si alloy by combined grain refinement and modification [J]. Materials Science and Engineering A, 2005, 395: 323-326.
[11] RAO A K, DAS K, MURTY B S, CHAKRABORTY M. Microstructural and wear behavior of hypoeutectic Al-Si alloy (LM25) grain refined and modified with Al-Ti-C-Sr master alloy [J]. Wear, 2006, 261: 133-139.
[12] LU L, DAHLE A K. Effects of combined additions of Sr and AlTiB grain refiners in hypoeutectic Al–Si foundry alloys [J]. Materials Science and Engineering A, 2006, 435/436: 288-296.
[13] SAGSTAD T, BHONDUS E. Master alloy for modification and grain refining of hypoeutectic and eutectic Al-Si foundry alloys: European, No. EP1134299 A1 [P]. 2001.
[14] EASTON M, St. JOHN D. Grain refinement of aluminum alloys [J]. Metallurgical and Materials Transaction A, 1999; 30A: 1613-1623.
[15] ZHAO Hong-liang, BAI Hui-long, WANG Jun, GUAN Shao-kang. Preparation of Al-Ti-C-Sr master alloys and their refining efficiency on A356 alloy [J]. Materials Characterization, 2009, 60: 377-383.
[16] ZHANG B Q, FANG H S, LU L, LAI M O, MA H T, LI J G. Synthesis mechanism of an Al-Ti-C grain refiner master alloy prepared by a new method [J]. Metallurgical and Materials Transactions A, 2003, 34A(8): 1727-1733.
[17] ABINASH B, WINFRIED R. Development of Al-Ti-C Grain Refiners Containing TiC [J]. Metallurgical Transactions A, 1986, 17A(12): 2127-2137.
[18] GREER A L, BUNN A M, TRONCHE A, EVANS P V and BRISTOW D J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al-Ti-B [J]. Acta Mater, 2000, 48: 2823-2835.
(Edited by FANG Jing-hua)
Corresponding author: ZHAO Hong-liang; Tel: +86-371-63887502; E-mail: zhlwkr@zzu.edu.cn
DOI: 10.1016/S1003-6326(09)60209-0


