Article ID: 1003-6326(2005)05-1045-04
Preparation of carbon nanotube composite material with metal matrix by electroplating
AN Bai-gang(安百刚)1, 2, LI Li-xiang(李莉香)2, LI Hong-xi(李洪锡)1
(1. State Key Laboratory for Corrosion and Protection of Metal, Institute of Metal Research,
Chinese Academy of Sciences, Shenyang 110016, China;
2. School of Chemical Engineering, Anshan University of Science and Technology, Anshan 114044, China)
Abstract:
It is demonstrated that the nickel can be deposited directly on the surface of carbon nanotubes without pre-sensitization by Sn2+ and Pd2+ in a watt bath containing suspended nanotubes by electroplating. The nickel is deposited as spherical nanoparticle on the nanotubes. By increasing reaction time, the carbon nanotube is fully coated with nickel. A probable model, which represents the formation process of carbon nanotube-nickel composites by electroplating, is presented. The results show that this method is efficient and simple for preparing carbon nanotube-metal composite.
Key words:
carbon nanaotubes; nickel; composite; electroplating CLC number: TG17;
Document code: A
1 INTRODUCTION
Carbon nanotube is expected to use in carbon nanotube-reinforced metal based composites, nanosized electronic devices, magnetic materials and area of catalysis, etc, due to its remarkable mechanical properties, unique metal-semiconductivity, special morphology and size[1-6]. Many researchers thus have dedicated to deposit metals or metal compounds on the surface of nanotube to prepare magnetic materials with high performance or to lower resistance metal-tube contacts[7-9]. A straightforward route to solve this problem is to melt elements on the surface of carbon nanotubes. However, only liquid with low surface tension will wet the surface of nanotubes[10, 11], which hinders its application. Electrochemical technology shows its flexibility in preparing metal composites and coatings. The techniques that coat nanotubes with Ni, Cu, Co by electroless plating have been reported by several authors[7, 12, 13], which need to carry out an activation-sensitization procedure to introduce catalytic nuclei on inert surface of the nanotubes, then metal is deposited on the surface of the nanotubes. While other metals such as Sn and Pd will also be introduced in composites or coatings. The present work reports a method in which multi-walled nanotubes-nickel composite is prepared through depositing nickel directly on the nanotubes without being pre-sensitized by Sn2+and Pd2+.
2 EXPERIMENTAL
The multi-walled carbon nanotubes were synthesized by catalytic decomposition of hydrocarbons using a floating catalyst method with a horizontal reaction chamber[14]. The main diameter of products was 50-70nm. The as-prepared specimen was suspended in distilled water and heat treated at 120℃ for 36h. Then the residual substance was heated in air at 520℃ for 1h. The black product was pre-cleaned in 12.8mol/L concentrated HNO3 for 12h and thoroughly washed with distilled water. The pre-cleaning preformed by nitric acid is to eliminate the residual metal catalyst particles from carbon nanotube preparation process. Moreover, the purification can modify the surface of carbon nanotube, and then can enhance the interfacial adhesion between carbon nanotube and metal film[15]. The purified carbon nanotubes were dried at 90℃ for 24h. The TEM image of purified nanotubes is shown in Fig.1.
The 500mg purified carbon nanotubes were taken into a 500mL watt plating bath and dispersed ultrasonically for 30min, which was as plating bath for preparing carbon nanotube-nickel composite. The composition of plating bath was as follows: NiSO4·6H2O 135g/L; NiCl2·H2O 28g/L; H3BO3 16g/L. All chemicals are of AP. A 20mm×20mm×1mm pure copper plate and 40mm×40mm×2mm pure nickel plate were used as cathode and anode respectively. The electroplating was carried out at a current density of 20A/dm2. The temperature of electroplating bath was maintained at 35-40℃ and pH of bath was held at 4.5. During electroplating, an air agitation with a rate of 10mL/s was held. The products deposited on cathode were peeled off and washed with distilled water, dried at 90℃ for 24h.

Fig.1 TEM image of purified multi-walled carbon nanotubes
A JSM6301F SEM and a Philips 420 TEM were used to characterize the deposited products.
3 RESULTS AND DISCUSSION
Carbon nanotubes have low chemical reactivity and a large curvature, which generally makes electroless plating be considered preferably as a method of coating nanotubes with metals. However, carbon nanotube also has good conductivity due to its unique structures, which motivates our attempts to deposit metals on sidewall of nanotubes by an electroplating method. We carried out an electroplating procedure in a watt bath containing suspended carbon nanotubes at 20A/dm2 current density. After 2min electroplating, it was found that the black deposits on the surface of cathode had been formed, and the black deposits were easy to be peeled off from the cathode. The SEM image of deposits on electrode is shown in Fig.2. Most of the carbon nanotubes are coated with the spherical nanoparticle, which indicates that the nickel tends to deposit as nanoparticles on the sidewall of nanotubes. The deposited spherical nickel nanoparticles may be due to the high curvature of nanotubes. On the curved surface, if the normal growth rate is higher than the lateral growth rate, the coating layer tends to be formed as spherical grains. It can be observed from the TEM image (Fig.3) that voids or gaps are also formed and the nanotubes are not completely and uniformly coated.

Fig.2 SEM image of deposits on cathode after 2min electroplating at 20A/dm2 current density
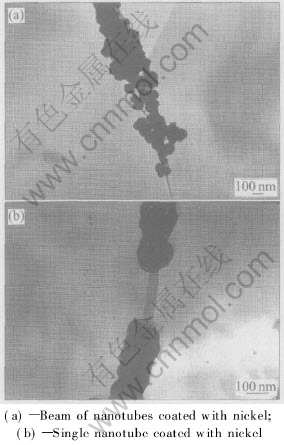
Fig.3 TEM images of carbon nanotubes coated with nickel
With increasing the electroplating time, the thickness of deposit layer progressively increases, leading to a sufficiently large grain size to ensure that grains may have several contact points to form continuous film. Fig.4 shows the morphologies of carbon nanotubes after 10min electroplating. The voids and gaps are eliminated by increasing the thickness of the deposit layer, and the better coating of nickel is obtained. Longer reaction time improves the surface of coating, but increases the diameter of the coated nanotubes.

Fig.4 SEM images of nickel-coated carbon nanotubes after 10min electroplating time
The results firstly show that the nickel can be deposited directly as nanoparticles on the nanotubes, even if carbon nanotubes are not sensitized and activated by Sn2+ and Pd2+. Furthermore, the uniform nickel coating on carbon nanotubes can be formed by electroplating. This may be attributed to the high concentration nanotubes suspended in plating bath, which forms a continuous net between the cathode and the anode. On the other hand, it is known that the nanotube is a good conductivity and has a good ability of electron transportation, thus the conductive net consisting of nanotubes can be formed between the electrodes. Because carboxylic and hydroxyl groups can be formed on the sidewall of carbon nanotubes during purifying processes of carbon nanotubes, the groups of carbon nanotubes may make hydrolysis nickel ions be adsorbed on the surface of nanotubes. With the migration of Ni2+ to cathode, Ni2+ adsorbed on the nanotubes may get electrons transported by nanotubes and is reduced to nanoparticles deposited on the nanotubes. Because of the existence of the double electronic layer, this is more probably to occur at the interface of cathode. Consequently, the more nanotubes coated with nickel are obtained near the cathode. The deposits with fiber and net morphology on the cathode are observed (Fig.4(a)). A probable model representing deposition process of nickel on the nanotubes is shown in Fig.5.
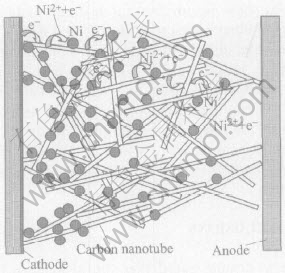
Fig.5 Schematic of electrons transportation by carbon nanotubes and deposition of nickel on
nanotubes in electroplating bath
It should be noticed that the nickel is directly deposited on the nanotubes by the present method, because there are no transition layers of Pd and Sn on the nanotubes as by electroless plating. The direct interaction of metal with nanotube is obtained (Fig.3(b)), thus the composites may have the better performance.
It is known that in three dimensional bulk materials, different metals exhibit different interaction with carbon. In general, the ability for the transition metals to bond with carbon atoms increases with the number of unfilled d-orbit. Metals with few d-vacancies such as Ni, Fe and Co exhibit finite solubility for carbon in certain temperature range. It is generally believed that the interactions of deposited metals with a graphite basal plane are weak[16, 17]. The interactions are suggested to be through the van der Waals forces and do not involve chemical bond formation between the metals and carbon atoms in the graphite basal plane. This should be due to the high chemical inertness of the ordered sp2 carbon network of graphite sheet. However, a carbon nanotube differs from a graphite sheet in their drastically different electronic structures, and the cylindrical sidewall of a nanotube is curved and in a non-planar sp2 bonding configuration. This may make a nanotube exhibit relatively higher chemical activity than a graphite sheet. After 2min electroplating, we obtain the carbon nanotubes coated with quasi-continuous nickel layer without any activation treatment, which may be due to the strong interaction of nickel with sidewall of nanotube. The strong interaction of nickel with sidewall of nanotube is attributed to the curvature-induced rehybridization of carbon sp2 orbits with the nickel d-orbits[18]. Memon et al[18] calculated the bonding configuration of nickel with the sidewall atoms on a single-walled carbon nanotube(SWNT), and compared the results with nickel bonding on a graphite sheet. The covalent bonding characteristic of nickel with carbon atoms on the nanotube was identified from the calculation. Zhang et al[19] directly deposited nickel on the surface of SWNTs by an electron-beam evaporation method and deduced covalent bonding characteristics of Ni-SWNTs interaction from significant condensation/sticking coefficient of nickel on SWNTs and low resistance ohmic contacts of SWNTs coated nickel. Therefore, the configuration between nickel and carbon atoms of the sidewall of nanotubes in carbon nanotubes-nickel composite, which is prepared by the present method, could have covalent bonding interaction.
4 CONCLUSIONS
It is demonstrated that the nickel can be deposited directly on the surface of carbon nanotubes without pre-sensitization by Sn2+ and Pd2+ in a watt bath containing suspended nanotubes by electroplating. The nickel is deposited as spherical nanoparticles on the nanotubes, consequently gaps and voids are formed on the coated nanotubes. However, with increasing reaction time, the nanotubes coated with continuous and uniform nickel can be obtained. Because the direct interaction of nickel with carbon atoms can be obtained by the present method, the carbon nanotubes-nickel composite may have the better performances. The results from this study indicate that the present method is efficient and simple for preparing carbon nanotube-metal composite and may be used to prepare a wide variety of carbon nanotubes-metal composites.
REFERENCES
[1]Tans S J, Vershueren A M R, Deeker C. Room-temperature transistor based on a single carbon nanotube [J]. Nature, 1998, 393(6680): 4952-4953.
[2]Saito R, Fujita M, Dresselhaus G, et al. Electronic-structure of chiral graphene tubels [J]. Appl Phys Lett, 1992, 60(18): 2204-2206.
[3]Treacy M M J, Ebessen T W, Gibson J M. Exceptionally high Youngs modulus observed for individual carbon nanotubes [J]. Nature, 1996, 381(6584): 678-680.
[4]Wong E W, Sheehan P E, Lieber C M. Nanobeam mechanics: elasticity, strength, and toughness of nanorods and nanotubes [J]. Science, 1997, 277(5334): 1971-1975.
[5]Jiang J, Dong J M, Xing D Y. Orbital motion of metallic carbon nanotubes in an axial magnetic field [J]. Appl Phys Lett, 2003, 83(26): 5515-5517.
[6]Planeix J M, Coustel N, Coq B, et al. Application of nanotubes as supports in heterogeneous catalysis [J]. J Am Chem Soc, 1994, 116(17): 7935-7936.
[7]Chen W X, Tu J P, Wang L Y, et al. Tribological application of carbon nanotubes in a metal-based composite coating and composites [J]. Carbon, 2003, 41(2): 215-222.
[8]Liu Y, Ling J, Wei U, et al. Effective synthesis of carbon-coated Co and Ni nanocrystallites with improved magnetic properties by AC arc discharge under an N-2 atmosphere [J]. Nanotechnology, 2004, 15(1): 43-47.
[9]Austin D W, Puretzky A A, Geohegan D B, et al. The electrodeposition of metal at metal/carbon nanotube junctions [J]. Chem Pyhs Lett, 2002, 361(5-6): 525-529.
[10]Ebbesen T W. Wetting, filling and decorating carbon nanotubes [J]. J Phys Chem Solids, 1996, 57(6-7): 951-955.
[11]Dujardin E, Ebbesen T W, Hiura H, et al. Capillarity and wetting of carbon nanotubes [J]. Science, 1994, 265(5180): 1850-1852.
[12]Ang L M, Hor T S A, Xu G Q, et al. Decoration of activated carbon nanotubes with copper and nickel [J]. Carbon, 2000, 38(3): 363-372.
[13]Chen X H, Xia J T, Peng J C, et al. Carbon-nanotube metal-matrix composites prepared by electroless plating [J]. Composites Science and Technology, 2000, 60(2): 301-306.
[14]Fan Y Y, Li F, Cheng H M, et al. Preparation, morphology, and microstructure of diameter-controllable vapor-grown carbon nanofibers [J]. J Mater Res, 1998, 13(8): 2342-2346.
[15]Esumi K, Ishigami M, Nakajima A, et al. Chemical treatment of carbon nanotubes [J]. Carbon, 1996, 34(2): 279-281.
[16]Ma Q, Rosenberg R. Interaction of Al clusters with the (0001) surface of highly oriented pyrolytic graphite [J]. Surf Sci, 1997, 391(1-3): L1224-L1229.
[17]Baumer M, Libuda J, Freund H. The temperature-dependent growth mode of nickel on the basal-plane of graphite [J]. Surf Sci, 1995, 327(3): 321-329.
[18]Menon M, Andriotis A, Froudakis G. Curvature dependence of the metal catalyst atom interaction with carbon nanotubes walls [J]. Chem Phys Lett, 2000, 320(5-6): 425-434.
[19]Zhang Y, Franklin N W, Chen R J, et al. Metal coating on suspended carbon nanotubes and its implication to metal-tube interaction [J]. Chem Phys Lett, 2000, 331(1): 35-41.
Foundation item: Project(2002AA327110) supported by the Hi-tech Research and Development Program of China
Received date: 2004-12-29; Accepted date: 2005-06-30
Correspondence: AN Bai-gang, PhD; Tel:+86-24-23893625; E-mail: bgan@imr.ac.cn


