
Density and viscosity of aqueous solution of K2CrO4/KOH mixed electrolytes
GUO Ya-jie(郭雅杰)1, 2, XU Hong-bin(徐红彬)2, GUO Fen(郭 奋)1,ZHENG Shi-li(郑诗礼)2, ZHANG Yi(张 懿)2
1. Research Center of the Ministry of Education for High Gravity Engineering and Technology,Beijing University of Chemical Technology, Beijing 100029, China;
2. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology,Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract:
The physicochemical properties are very important in theoretical investigation of aqueous electrolyte solution and industrial design of hydrometallurgical processes. In the green hydrometallurgical process of chromite ore with sub-molten salt medium of KOH, the ternary system of KOH+K2CrO4+H2O is essential to process control and industrial operation. In order to satisfy the needs of both fundamental research and industrial application, the dynamic viscosity (η) and density (ρ) of mixed aqueous electrolyte solution of KOH and K2CrO4 were measured over a temperature range from 15 to 60 ℃ by using Ubbelohde-type capillary viscometers and a series of densimeters, respectively. The temperature is controlled to an accuracy of ±0.01 ℃ throughout the experiment with thermostat. The dynamic viscosity and density of the ternary systems are performed as functions of chromate and hydroxide concentration and temperature. The regression equations for viscosity and density are obtained with a least-square method and the calculated values are consistent well with the experimental data. The semi-empirical equation obtained will be helpful and instructive to industrial application.
Key words:
viscosity; density; potassium chromate; potassium hydroxide; mixed electrolytes;
____________________________________________________________________________________________
1 IntroductionThe green hydrometallurgical process of chromite ore with sub-molten salt medium is proposed and developed to solve the serious environmental problem in the traditional production process of chromate[1-2]. In the green process, the efficient separation of K2CrO4 from the multi-component electrolytes system of K2CrO4-KOH-KAlO2-K2SiO3-K2MnO4-H2O is very important[2-5]. As for both fundamental research and industrial application, the needs for physicochemical property of the multiple-solute electrolyte solution become imperative and urgent. The ternary system of KOH+K2CrO4+H2O studied in this work is one of the sub-systems of the mixed electrolytes. However, to the authors’ knowledge, the physicochemical property data of this ternary aqueous electrolyte have seldom been reported so far.
The density is one of the key thermodynamic properties of electrolyte solutions and belongs to an equilibrium property, while the viscosity is one of the key transport properties of electrolyte solutions and belongs to a dynamic state property. Both of them are indispensable basic data to engineering design and process optimization[6-8]. In the present work, the dynamic viscosity (η) and density (ρ) of the ternary system of KOH+K2CrO4+H2O were measured and the experimental data as a function of chromate and hydroxide concentration over the temperature range from 15 to 60 ℃ were regressed. A semi-empirical equation describing the relationship between the viscosity or density of the solutions as well as the primary variables was obtained.
2 Experimental2.1 Apparatus and reagents
The main apparatus used in this work included a thermostat (type JWC-32C1, Shanghai S.R.D Scientific Instrument Co. Ltd.) with a constant water circulation for maintaining constant temperature throughout the experiment to an accuracy of ±0.01 ℃, an electronic analytical balance (Mettler AE240) with an uncertainty of 0.1 mg, an electronic digital stopwatch correct to ±0.01 s, a series of densimeters with an accuracy up to 0.1 mg/cm3 and Ubbelohde capillary viscometer (type 1836-A, Shanghai Liangjing Glass Instruments Factory, China) with a diameter of 0.46 mm.
The chemical reagents potassium chromate (K2CrO4) and potassium hydroxide (KOH) used in this work were AR grade and GR grade, respectively, and used directly without further purification (potassium chromate, AR, ≥98.5% (mass fraction); potassium hydroxide, GR, ≥85.0% (mass fraction)). The pure water used throughout the experiments was double-distilled and its conductivity was lower than 0.1 mS/m.
The solutions consisting of individual samples with total volume of 1 L were prepared on the basis of mass, using the analytical balance. The chromate and hydroxide concentration were determined by titration with (NH4)2Fe(SO4)2 and HCl standard solution in the presence of indicator of n-phenylanthranilic acid and phenolphthalain, respectively.
2.2 Density measurement
The density measurements were carried out by a series of densimeters. A suitable densimeter was selected and then immersed in the solution. After the solution temperature reached a desired point, the density value was read from the meniscus of the solution with the densimeter.
2.3 Viscosity measurement
The viscosity (η) was measured using a Ubbelohde capillary viscometer, which was placed inside the thermostat. The viscometer was calibrated with high- purity water at 15, 25, 30, 40, 50 and 60 ℃, and was thoroughly cleaned and perfectly dried, then filled with experimental solutions. After thermal equilibrium had been achieved at the required temperature, the times of the flow of the solutions and water were recorded with the electronic digital stopwatch. At least five repetitions of each datum point obtained were reproducible to ±0.05 s for solutions and ±0.02 s for water, and the results were averaged. The viscosity (η) was then calculated according to Eq.(1)[7-8]
![]() (1)
(1)
where η (mPa·s), ρ (g·cm-3), t (s), ηw (mPa?s), ρw (g·cm-3), tw (s) represent the viscosity, density and flow time of the mixture and water, respectively. The densities and viscosities of pure water were taken from Data Book of Chemistry & Chemical Industry Physical Properties (Inorganic compounds)[9].
In order to improve the accuracy, tw was determined daily before and after the test solution measurements[10]. From several hundred independent runs, the results show that the efflux times for water drop over a period of about five months. This variation is consistent with a very slow dissolution of the glass in the caustic solutions causing a small but noticeably increase in the capillary bore. This dissolution is sufficiently slow and has no practical effect on the measured viscosity.
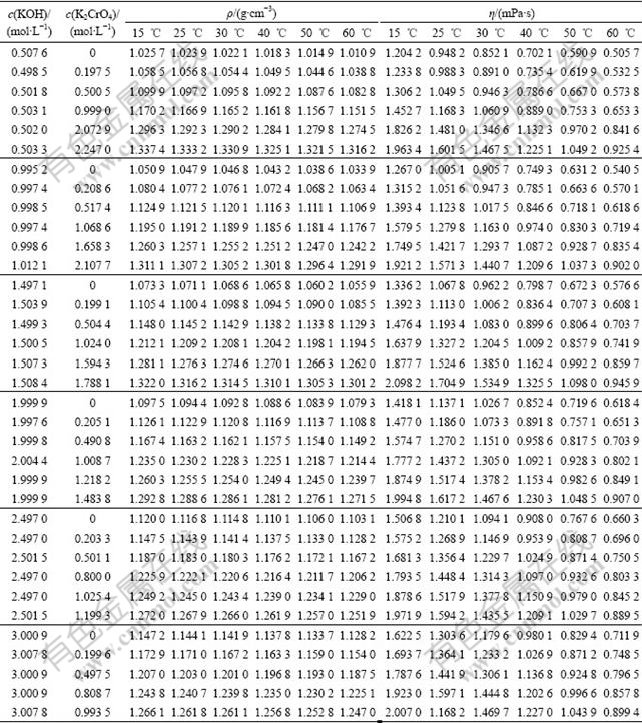
3 Results and discussionThe experimental densities and viscosities for KOH+K2CrO4+H2O ternary system at the temperatures of 15, 25, 30, 40, 50 and 60 ℃ are listed in Table 1. From Table 1, the densities decrease with increasing temperature at fixed chromate and hydroxide concentration and increase with increasing chromate or hydroxide concentration at constant temperature. The density variation is due to the expansion of the volume of the solution with the increasing temperature of the system. The same occurs at the viscosities of the system. This results show that strong interactions between the solute species and water molecules exist in the solution and that this interaction weakens with increasing temperature.
Table 1 Experimental densities (ρ) and viscosities (η) of ternary system (KOH+K2CrO4+H2O) as a function of chromate and hydroxide concentration from 15 ℃ to 60 ℃
3.1 Correlation of densities
The effects of temperature and concentration on densities are shown in Figs.1 and 2. As can be seen, the mixture densities display satisfactorily linear dependence (with R2>0.995) on temperature and solute concentration.
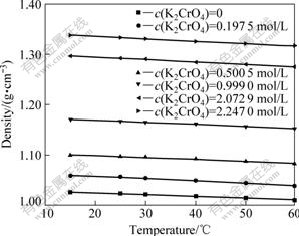
Fig.1 Effect of temperature on solution density at KOH of 0.5 mol/L
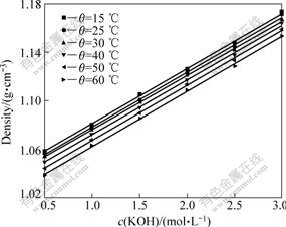
Fig.2 Effect of hydroxide concentration on solution density under different temperatures at K2CrO4 of 0.2 mol/L
According to this law, the experimental data were regressed by Matlab 6.5 with the least-squares method[11-15]. The regression equation describing the relationship between the solution densities and basic variables (temperature, chromate and hydroxide concentration) is
ρ=1.019 8-4×10-4θ+0.043 5c(KOH)+0.128 3c(K2CrO4) (2)
where ρ (g/cm3), θ (℃), c(KOH) (mol/L) and c(K2CrO4) (mol/L) represent the density, temperature, chromate and hydroxide concentration in terms of molality of the solution, respectively.
Some of the calculated values and relative errors are listed in Table 2. This semi-empirical equation enables to predict the densities of KOH+K2CrO4+H2O ternary system over the ranges of 15 ℃≤θ≤60 ℃, 0.5 mol/L≤c(KOH)≤3 mol/L and 0≤c(K2CrO4)<Saturation, with average error of 0.3410% and a maximum error of 1.3159% under conditions of θ=60 ℃, c(KOH)=1.012 1mol/L and c(K2CrO4) = 2.107 7 mol/L.
3.2 Correlation of viscosities
The effects of the three independent variables on mixture viscosity were investigated using the nonlinear regression method in Origin 8.0. The results show that the nature logarithm of the mixture viscosity display satisfactorily linear dependence (R2>0.995) on chromate and hydroxide concentration, and the curves of dynamic viscosity vs temperature accord with second-order polynomial equations (R2>0.999) (see Figs.3 and 4).
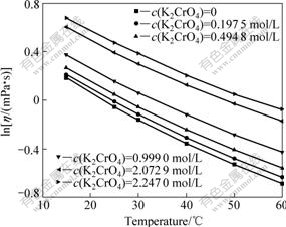
Fig.3 Effect of temperature on solution viscosity at KOH of 0.5 mol/L
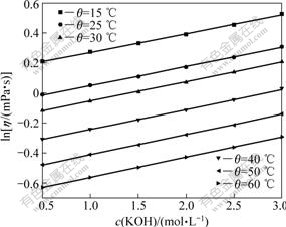
Fig.4 Effect of hydroxide concentration on solution viscosity under different temperature at K2CrO4 of 0.2 mol/L
The regression equation for viscosity obtained by the least-squares method[11-13, 15] is
η=exp[0.430 0-0.025 1θ+1×10-4θ2+0.130 7c(KOH)+0.236 6c(K2CrO4)] (3)
where η (mPa·s) represents the viscosity of the solution.
Some of the calculated viscosity values are presented in Table 2. The average error between the calculated and the experimental data is 1.1447% with a maximum of 7.669 0% at θ=40 ℃, c(KOH)=1.508 4 mol/L and c(K2CrO4)=1.788 1 mol/L and the others are all below 5.8%. This semi-empirical equation for viscosity can meet the needs for industrial application.
Table 2 Comparison of experimental densities and viscosities of KOH+K2CrO4+H2O with calculated data
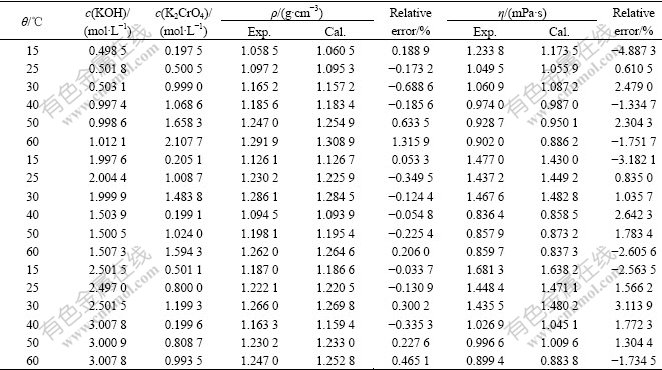
1) Regression equations for viscosity and density are obtained with the least-squares method and the average errors between the calculated and experimental data are 0.341 0% for densities (with maximum value of 1.315 9%) and 1.144 7% (with maximum value of 7.669 0%) for viscosities, respectively.
2) The semi-empirical equations obtained can be used over the range of experimental conditions (15 ℃≤θ≤60 ℃, 0.5 mol/L≤c(KOH)≤3 mol/L and 0≤ c(K2CrO4)<Saturation) and will be helpful and instructive to industrial application.
References[1] QI T, ZHANG Y, GUO Y H, LI Z H, WANG Z K. The new chemical process for zero emission of chromium-containing residue [J]. Chinese Journal of Chemical Metallurgy, 1999, 20(1): 92-97. (in Chinese)
[2] ZHENG S L, ZHANG Y, LI Z, QI T, LI H Q, XU H B. Green metallurgical processing of chromite [J]. Hydrometallurgy, 2006, 82: 157-163.
[3] XU H B, ZHANG Y, YOU H X. Separation of potassium chromate by salting-out crystallization [J]. Journal of Chemical Industry and Engineering, 2007, 58(4): 930-937. (in Chinese)
[4] WANG J, XU H B, ZHANG S P, ZHANG Y. Experimental investigation on separation of potassium chromate from its aqueous solution by solventing-out [J]. The Chinese Journal of Process Engineering, 2007, 7(2): 246-251. (in Chinese)
[5] QU J K, XU H B, ZHENG S L, QI T, ZHANG Y. Separation of impurities from liquid phase oxidation product of chromite and recycle of caustic potash solution [J]. Hydrometallurgy of China, 2007, 26(4): 193-197. (in Chinese)
[6] LI X X, LIU Y X, WEI X H. Density, viscosity and surface tension at 293.15 K and liquid-liquid equilibria from 301.15 K to 363.15 K under atmospheric pressure for the binary mixture of diethylene glycol diethyl ether + water [J]. Chem Eng Data, 2004, 49(4): 1043-1045.
[7] WANG L C, XU H S, ZHAO J H, SONG C Y, WANG F A. Densities and viscosities of Niacin + 3-Picoline + water mixture from (293.15 to 343.15) K [J]. Chem Eng Data, 2005, 50(1): 254-257.
[8] ZHANG P, WANG F A, WANG J Y, LI C W, REN B Z. Volumetric and viscosity behaviors of the chromic anhydride+sodium dichromate+water from 298.15 to 333.15 K [J]. Journal of Molecular Liquids, 2008, 142: 22-28.
[9] LIU G Q, MA L X, LIU J. Data book of chemistry and chemical industry physical properties (Inorganic compounds) [M]. Beijing: Chemical Industry Press, 2002: 3-12. (in Chinese)
[10] SIPOS P, STANLEY A, BEVIS S, HEFER G, PETER M. Viscosities and densities of concentrated aqueous NaOH/NaAl(OH)4 mixtures at 25 ℃ [J]. Chem Eng Data, 2001, 46(3): 657-661.
[11] LI F Y, LI Y C. Measurement of densities and viscosities for mixed solutions of FeSO4-Fe2(SO4)3-H2SO4 [J]. Chemical Engineering, 2006, 34(4): 39-42. (in Chinese)
[12] WANG M R. Matlab and scientific computing [M]. Beijing: Electronics Industry Press, 2003: 219-229. (in Chinese)
[13] LI J, PRESTIGE C A, ADDAI-MENSAH J. Viscosity, density and refractive index of aqueous sodium and potassium aluminate solutions [J]. Chem Eng Data, 2000, 45(4): 665-671.
[14] GALLEGUILLOS H R, MOLINA M A, GRABER T A, TABOADA M E. Experimental determination of densities of aqueous electrolyte mixtures containing B(OH)3 or Na2B4O7 and their correlation with pitzer model [J]. Ind Eng Chem Res, 2006, 45(19): 6604-6613.
[15] de LUCAS A, DONATE M, RODRIGUEZ J F. Vapor pressures, densities, and viscosities of the (water + lithium bromide + sodium formate) system and (water + lithium bromide + potassium formate) system [J]. Chem Eng Data, 2003, 48(1): 18-22.
_________________________
Foundation item: Project(2007CB613501) supported by the National Basic Research Program of China; project(50904058) supported by the National Natural Science Foundation of China; project(2006BAC02A05) supported by National Science and Technology Pillar Program of China
Corresponding author: XU Hong-Bin; Tel: +86-10-82627096; E-mail: ipecas_hbxu@263.net


