网络首发时间: 2013-12-27 16:34
化学镀Ni改性Li4Ti5O12材料的制备及其电化学性能研究
广东工业大学轻工化工学院
摘 要:
为提高Li4Ti5O12的电化学性能和防气胀性能, 结合固相法并用化学镀金属Ni对样品进行表面修饰改性, 制备了Li4Ti5O12/Ni复合材料。使用X射线衍射 (XRD) 、Raman光谱、扫描电镜 (SEM) 对样品进行了物相分析和形貌表征, 结果显示:合成的样品具有单一的尖晶石结构, Li4Ti5O12/Ni复合材料和Li4Ti5O12在拉曼光谱图上具有相同的频率振动特性, 说明化学镀法表面修饰Li4Ti5O12/Ni复合材料并没有改变纯相Li4Ti5O12的结构, 且样品的颗粒大小均匀、形状规则, 颗粒尺寸在0.51.0μm范围内。采用充放电测试、循环伏安和交流阻抗对样品的电化学性能进行表征, 结果表明:Li4Ti5O12/Ni复合材料在1C和5C倍率下首次放电比容量分别为149.8和132.5 mAh·g-1, 循环50次后分别为144.1和124.0 mAh·g-1, 容量保持率为96.2%和93.6%, 表现出良好的倍率性能和循环性能, Li4Ti5O12/Ni的阻抗值明显小于纯相Li4Ti5O12。
关键词:
锂离子电池;负极材料;Li4Ti5O12/Ni;电化学性能;
中图分类号: TM912.9
作者简介:李军 (1975-) , 男, 湖南邵阳人, 博士, 副教授, 研究方向:电池材料;电话:13826078556;E-mail:gdutlijun@163.com;
收稿日期:2013-07-12
基金:国家自然科学基金项目 (20672023);广州市科技计划项目 (2011Y1-00010);广东省科技计划项目 (2012B050100010) 资助;
Synthesis of Modified Li4Ti5O12 Anode Material with Electroless Plating Nickel and Its Electrochemical Properties
Li Jun Huang Jiwei Zhou Yan Tao Xun
School of Chemical Engineering, Guangdong University of Technology
Abstract:
In order to improve electrochemical and anti-flatulence properties of Li5Ti5O21, microscopy Li4Ti5O12/ Ni ( LTO / Ni) anode materials were prepared through high temperature solid-state reaction by modifying the surface of samples via facile electroless deposition of Ni. The microstructure and morphology of the prepared samples were characterized by X-ray diffraction ( XRD) , Raman spectroscopy and scanning electron ( SEM) . The results showed that the samples possessed spine structure, it could be observed that the Li4Ti5O12/ Ni composite and Li4Ti5O12shared the same vibrating frequency, which indicated that the addition of Ni had no effect on the structure of pure Li4Ti5O12, and the particles were uniform and were regularly shaped within 0. 5 1. 0 μm. Electrochemical properties of the samples were evaluated by the charging and discharging tests, cyclic voltammograms and AC impedance spectroscopy. The results indicated that the Li4Ti5O12/ Ni composite exhibited good rate performance and cycle performance. At the discharge rate of 1C and 5C, their specific capacities were 149. 8 and 132. 5 mAh·g- 1and remained at 144. 1 and 124. 0 mAh·g- 1respectively after 50 cycles, with a retention rate of capacity of 96. 2% and 93. 6% indicating good rate capacity and cycle performance. And the resistance value of Li4Ti5O12/ Ni was much lower than that of pure Li4Ti5O12material.
Keyword:
lithium-ion batteries; anode materials; Li4Ti5O12/ Ni; electrochemical properties;
Received: 2013-07-12
尖晶石型Li4Ti5O12在锂离子的嵌入和脱出过程中其晶格结构基本不发生变化, 被称为“零应变”材料[1 - 2], 正是这种独特的性能特点使得Li4Ti5O12负极材料具有优异的结构稳定性, 并体现出良好的循环稳定性和平稳的电压平台。同时, Li4Ti5O12有着较高的对锂电位 ( 1. 55 V vs. Li/Li+) , 不易引起金属锂的析出, 能够在大多数液体电解质的稳定电压区间使用, 避免SEI膜的产生, 被认为是锂离子电池理想的负极候选材料[3], 作为动力锂离子电池和储能锂离子电池的负极材料有着非常广阔的应用前景。然而, Li4Ti5O12材料本身具有较低的电导率 ( 1 × 10- 9S·cm- 1) , 这也正是当前研究的重点[4 - 7]。当前, 向Li4Ti5O12表面引入电子导电率高的物相, 包覆或分散在颗粒表面以增强主相颗粒间的电子导电能力, 提高材料的导电性能, 是改善材料倍率性能和循环性能的有效方法[8 - 13]。碳包覆和制备金属及金属氧化物复合材料虽然能提高导电性能, 大电流充放电性能得到改善, 但是难以在Li4Ti5O12材料表面掺杂或包覆均匀, 从而导致性能不稳定。本文结合二步高温固相反应法, 采用化学镀金属Ni对样品进行表面修饰改性, 制备了Li4Ti5O12/ Ni复合材料, 该方法使Li4Ti5O12粉末与镀液充分接触而发生反应, 金属Ni高度分散于Li4Ti5O12粉末表面, 形成细致光亮的镍镀层, 与Li4Ti5O12粉末结合更加紧密牢固, 从而提高材料的倍率性能和循环性能。
1 实验
1. 1 试样制备
按一定的化学计量比准确称取Ti O2 ( 分析纯) 、Li2CO3 ( 电池级) ( 其中摩尔比Li∶ Ti = 0. 84∶ 1. 00) , 原料混合在无水乙醇 ( 分析纯) 中球磨4 h后取出于120 ℃下真空干燥12 h, 将前驱体研磨后盛放在坩埚内然后转移至马弗炉中, 600 ℃ 下保温4 h继续升温至800 ℃的空气气氛下烧结12 h ( 升温速率5 ℃·min- 1) , 随炉冷却至室温即得到样品Li4Ti5O12粉末, 记为LTO。
将LTO粉末用Pd活化液充分润湿, 然后倒入化学镀镍液, 其中硫酸镍的溶度为25 g·L- 1, 混合均匀后, 置于80 ℃ 下磁力搅拌反应, 待溶液变灰色, 即停止, 然后过滤, 洗涤, 真空干燥即得到LTO / Ni复合材料。
其中Pd活化液的配制: 在50 ml 0. 1 mol的十二烷基磺酸钠溶液中加入0. 5 ml HNO3, 溶液的p H约为1. 0 ~ 1. 5, 再加入0. 24 mmol乙酸钯 ( Pd ( OAc) 2) , 室温下不断搅拌使其溶解, 5 min后溶液呈橙色, 不断搅拌并缓慢加入0. 5 ml 10% 的肼水于上述溶液, 溶液呈深棕色。
1. 2 样品表征
用日本理学D/MAX-PC2200 X射线衍射仪 ( Cu钯, λ =0. 15405 nm) 作物相分析, 电压40 k V, 电流20 m A, 扫描范围为10° ~ 70°, 扫描速度8 ( °) ·min- 1;HORIBA Jobin Yvon激光紫外拉曼光谱仪, 激发光源: Ar+, 488 nm, 400 m W; Hitachi S-550 型扫描电子显微镜 ( SEM) 观察合成产物的形貌。
1. 3 电化学性能测试
模拟试验电池正极片按质量比Li4Ti5O12∶ 乙炔黑∶ PVDF = 8∶ 1∶ 1 混合均匀后经压片制成, 将制好的电极片于120 ℃真空干燥24 h。负极为金属锂片、隔膜为celgard2400 聚丙烯多孔膜, 以1. 0 mol·L- 1六氟磷酸锂 ( Li PF6) /碳酸乙烯酯 ( EC) 、碳酸二甲酯 ( DMC) 和碳酸甲乙酯 ( EMC) ( 质量比1∶ 1∶ 1) 为电解液。所有电池的装配过程均在充满氩气的手套箱中进行。将试验电池置于新威BTS计算机程控充放电测试仪上作电化学性能测试, 电压区间为1. 0 ~ 2. 5 V, 测试的放电倍率分别约为0. 2C, 1. 0C, 2. 0C和5. 0C。
采用PGZ301 型电化学工作站 ( 法国Radiometer Analytical SAS公司) 对模拟电池进行循环伏安测试以及电化学阻抗谱分析。其中, 循环伏安的扫描电压范围为1. 0 ~2. 5 V, 扫描速率为0. 1 m V·s- 1: 电化学阻抗分析过程中对模拟电池施加的交流信号的振幅为5 m V, 频率测试范围0. 01 Hz ~ 100 k Hz。
2 结果与讨论
2. 1 LTO和LTO / Ni的样品结构和形貌特征
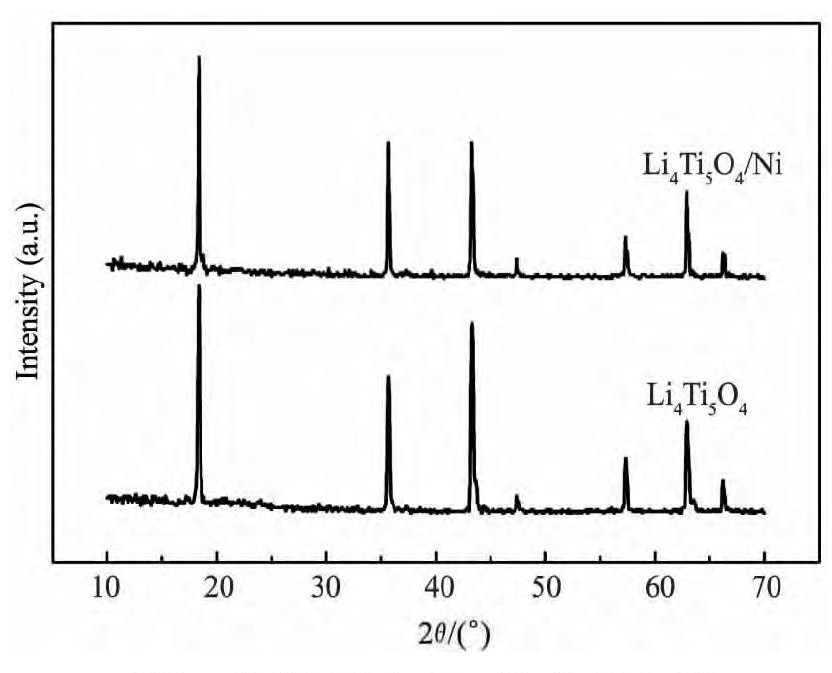
图1 为样品LTO和LTO/Ni的XRD图, 从图1可以看出, 两组样品的XRD主要衍射峰与标准JCPDS卡 ( PDF#49-0207) 相吻合, 没有杂质峰的出现, 显示出完好的尖晶石结构, 峰形尖锐, 具有均一的立方相, 结晶性能良好。
图1 样品LTO和LTO/Ni的XRD图Fig. 1 XRD patterns of LTO and LTO / Ni samples
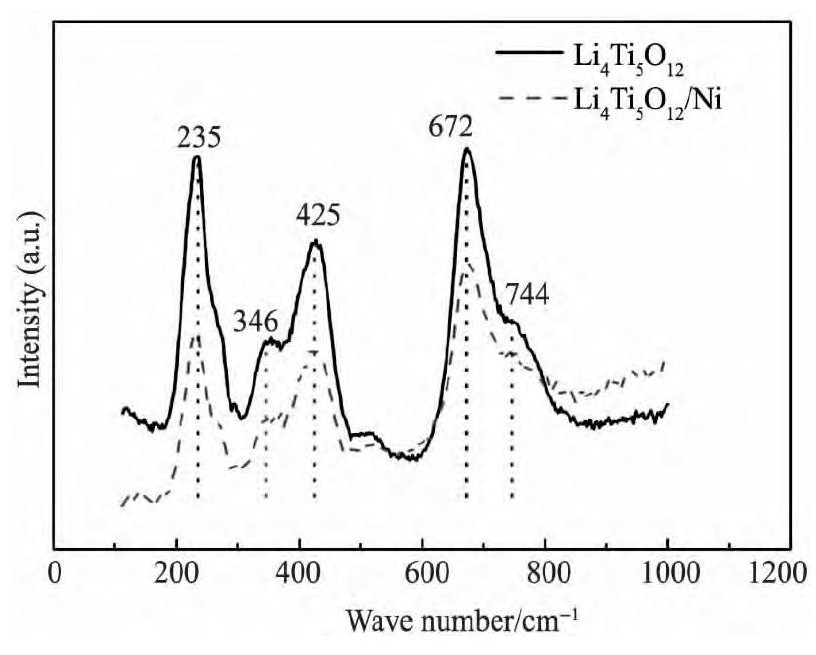
图2 样品LTO和LTO/Ni的拉曼光谱图Fig. 2 Raman spectra of LTO and LTO / Ni samples
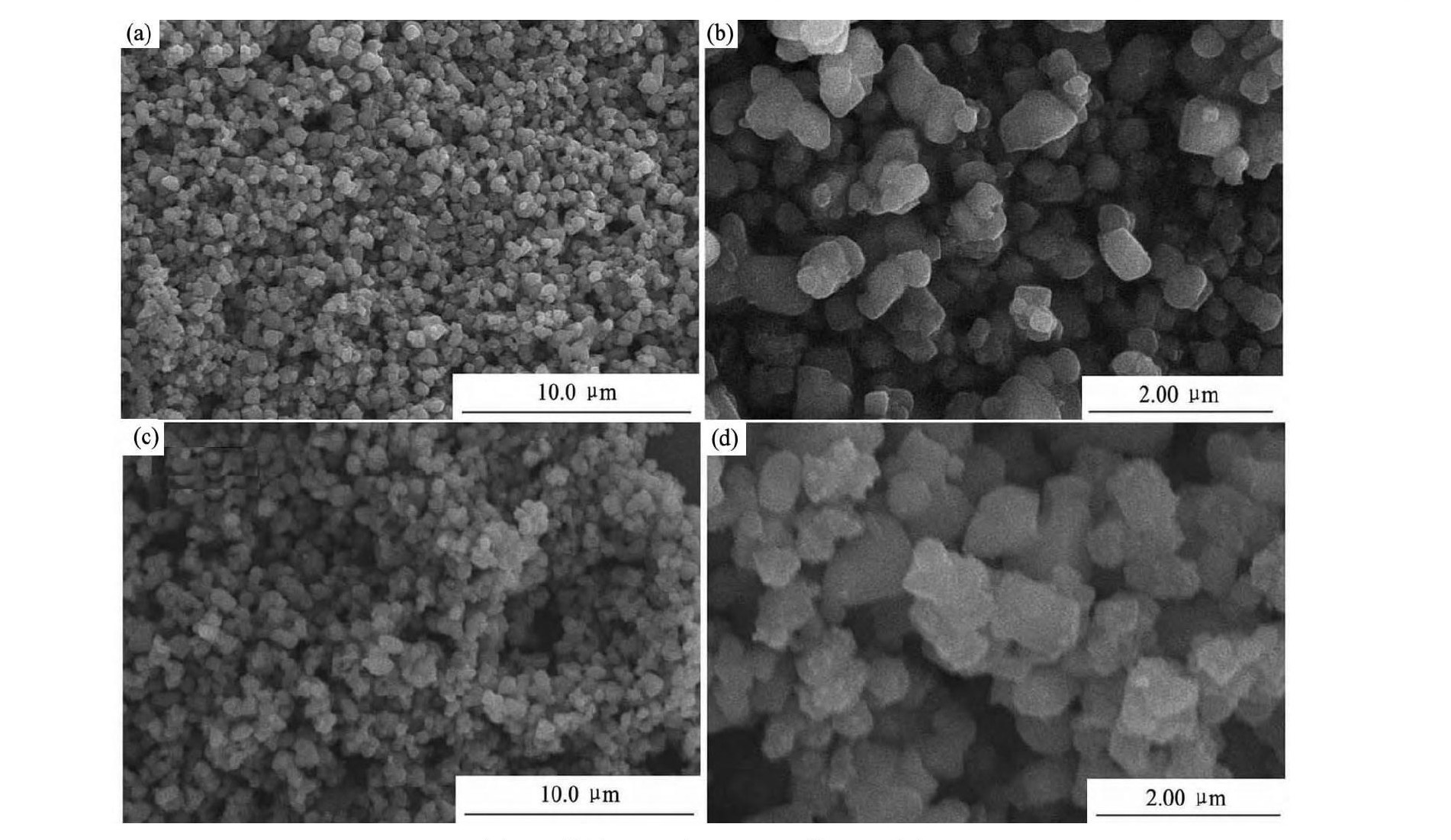
图2 为样品LTO和LTO/Ni的拉曼光谱图。从图2 可以观察两组曲线在0 ~ 1000 cm- 1中存在5个强烈的振动峰, 分别处于235, 346, 425, 672 和744 cm- 1。在672 和744 cm- 1两个高频区对应着Ti O6正八面体中Ti - O键的振动, 在中频区300 ~500 cm- 1对应着Li O4与Li O6多面体中Li - O键的伸缩振动[14]。LTO/Ni复合材料和LTO在拉曼光谱图上具有相同的频率振动特性, 说明化学镀法表面修饰LTO/Ni复合材料并没有改变纯相LTO的结构。图3 为样品LTO和LTO/Ni的SEM照片, 放大倍数为5000 和20000 倍, 由图3 可看出, 样品LTO和LTO/Ni形貌都非常相似, 颗粒大小均匀且形状规则, 颗粒尺寸在0. 5 ~ 1. 0 μm范围内。两种试样都有轻微的团聚现象。
2. 2 电化学性能
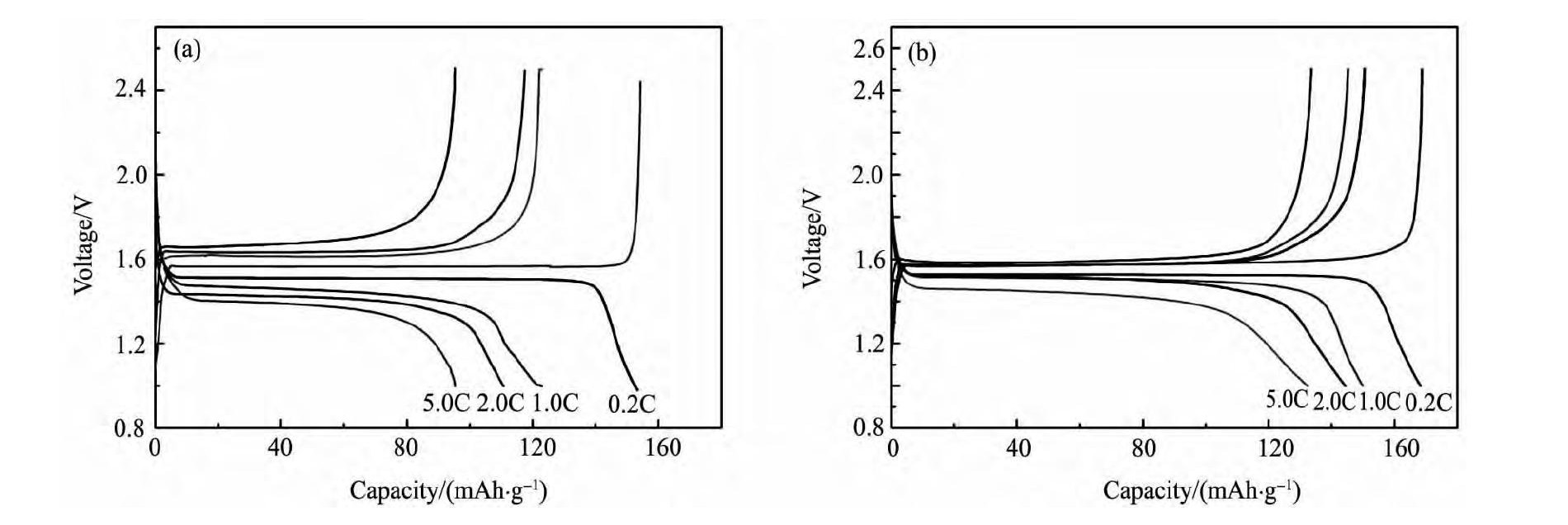
图4 示出样品LTO和LTO/Ni复合材料的充放电性能曲线。由图4 可见, 各倍率下, LTO/Ni复合材料的比容量均高于LTO材料, LTO /Ni复合材料在0. 2C, 1. 0C, 2. 0C的倍率下, 放电电压平台均保持在1. 5 V以上, 随着充放电倍率的增大 ( 从0. 2C到2. 0C) , LTO / Ni复合材料的比容量逐渐下降, 在0. 2C, 1. 0C, 2. 0C的放电倍率下, 首轮放电比容量分别为168. 8, 149. 8, 144. 3 m Ah·g- 1, 平台容量为总容量的90% 以上; 当放电倍率进一步提高到5C时, 放电平台出现了比较明显的下降, 平台电压为1. 46 V, 放电比容量为132. 5 m Ah·g- 1, 平台容量占总容量的80% 以上。总的来说, Li4Ti5O12/Ni复合材料明显的改善了材料的高倍率性能。
图3 样品LTO和LTO/Ni的SEM图Fig. 3 SEM images of LTO ( a, b) and LTO / Ni ( c, d) samples
图 4 不同倍率下 LTO 和 LTO/Ni 的首次充放电曲线Fig. 4 Charge / discharge curves for LTO ( a) and LTO / Ni ( b) samples at 0. 2C,1. 0C,2. 0C and 5. 0C
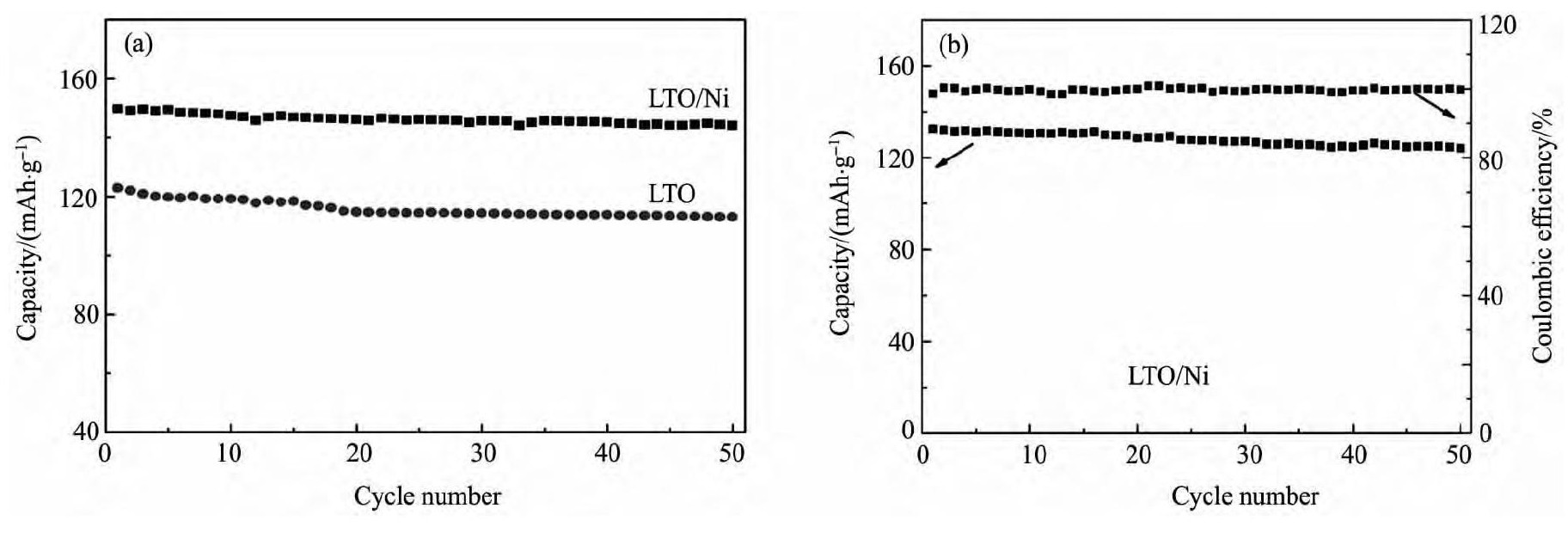
图5 为实验样品LTO和LTO/Ni在1. 0C倍率下的循环性能曲线, 从图5 中可以看出, 在1. 0C放电时, 循环50 次后, LTO/Ni的比容量为144. 1 m Ah·g- 1, 远高于同倍率下LTO的113. 1 m Ah·g- 1, 容量保持率分别为96. 2% 和92. 3% ; 5. 0C时, 材料的放电比容量为132. 5 m Ah·g- 1, 循环50 次后容量下降为124. 0 m Ah·g- 1。结果表明, LTO/Ni具有更优越的循环稳定性。
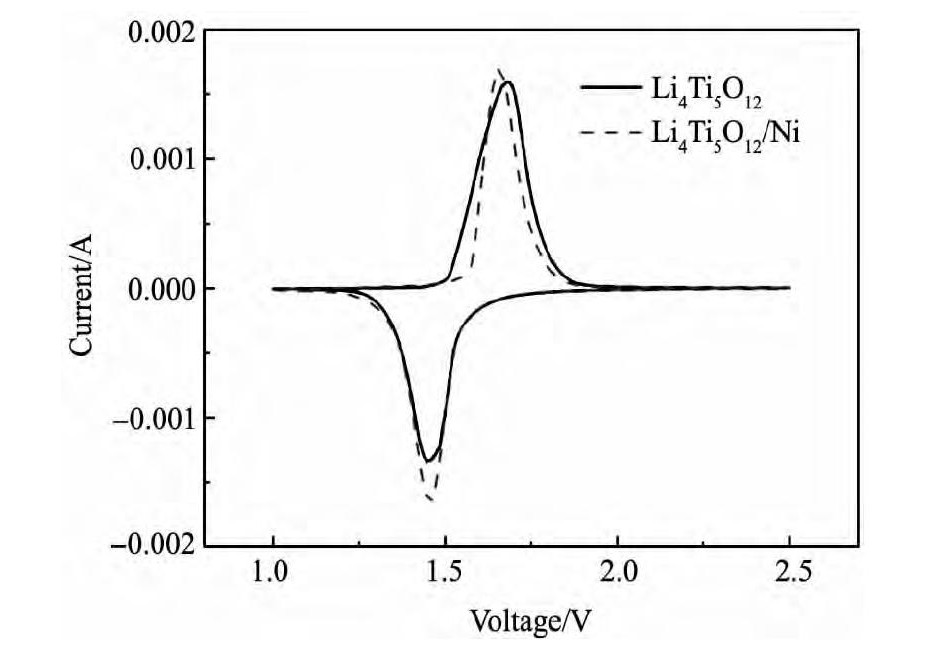
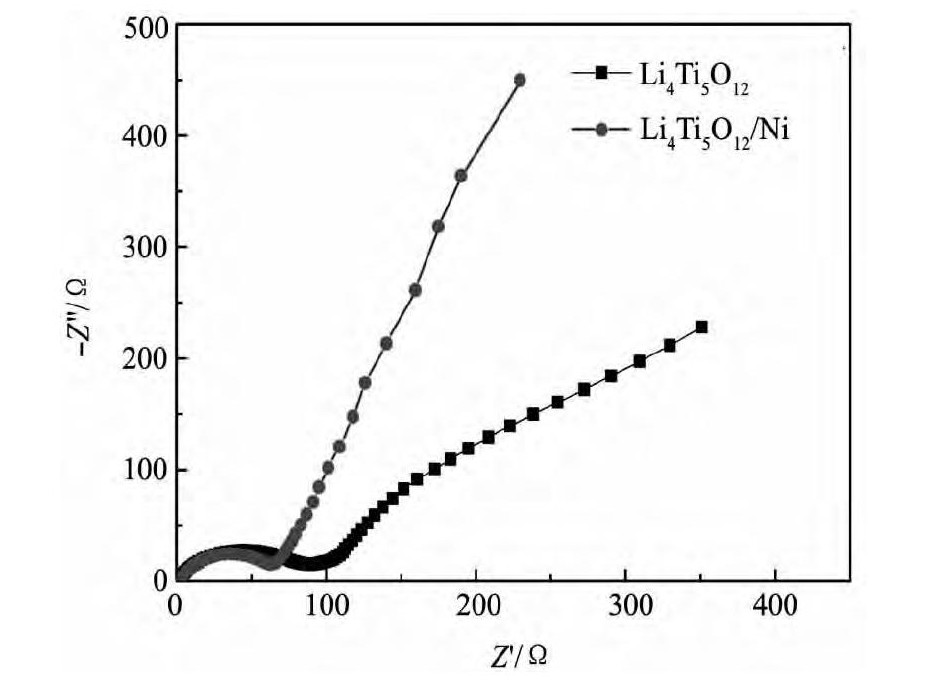
图6 为样品LTO和LTO/Ni复合材料在扫描速度0. 1 m V·s- 1下循环伏安曲线, 从图6 可以看出, 两组曲线在1. 0 ~ 2. 5 V之间都只有一组氧化还原峰, 说明样品LTO和LTO/Ni脱嵌锂反应无中间相生成, 且峰面积近似相等, 具有较高的库仑效率。对LTO/Ni复合材料阳极氧化峰电位约为1. 66 V, 阴极还原峰电位约为1. 46 V, 氧化还原峰间距差值约0. 20 V, 比LTO材料的峰间距 ( 0. 24V) 小, 这说明LTO / Ni复合材料的可逆性要好, 电化学极化小, Li+的扩散性能较好。图7 为样品LTO和LTO / Ni复合材料的Nyquist曲线, 在高频区呈现半圆形, 对应着的是电极电化学反应过程, 它代表了电荷转移过程的阻抗以及电极和电解液间的界面容抗; 而在低频区为一条斜线, 对应着的是电极的扩散过程, 代表锂离子扩散至宿主晶格过程中所引起的Warburg阻抗[15], LTO/Ni的阻抗值明显小于LTO。
图5 不同倍率下的循环性能曲线Fig. 5 Curves of cycle performances of LTO and LTO / Ni samples at 1. 0C ( a) and 5. 0C ( b)
图6 样品LTO和LTO/Ni的循环伏安曲线Fig. 6 Curves of cyclic voltammogram of LTO and LTO / Ni sam-ples
图7 样品LTO和LTO/Ni的交流阻抗图Fig. 7Electrochemical impedance spectroscopy of LTO and LTO / Ni samples
3 结论
结合二步高温固相法, 采用化学镀表面修饰改性制备了LTO/Ni复合材料, 考察了金属Ni表面修饰改性对LTO晶体结构、粒径大小、形貌和电化学性能的影响。结果表明, LTO/Ni复合材料具有良好的结晶度和高相纯度, 粒径分布均匀, 约为0. 5 ~ 1. 0 μm。相比于Li4Ti5O12, LTO/Ni复合材料导电性提高, 充放电容量有所增大, 循环性能得到了很大提高, 1. 0C时达到了149. 8 m Ah·g- 1, 5. 0C时, 比容量为132. 5 m Ah·g- 1, 1. 0C循环50次后容量保持率为96. 2% , 循环伏安曲线与交流阻抗测试表明, LTO/Ni复合材料的可逆性要好, 电化学极化小, Li+的扩散性能较好。
参考文献