
Extraction of indium from indium-zinc concentrates
LI Shi-qing(李仕庆)1, 2, TANG Mo-tang(唐谟堂)1, HE Jing(何 静)1,
YANG Sheng-hai(杨声海)1, TANG Chao-bo(唐朝波)1, CHEN Yong-ming(陈永明)1
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Liuzhou Huaxi Group Corporation, Liuzhou 545006, China
Received 20 January 2006; accepted 19 June 2006
Abstract:
A new process for extracting indium from indium-zinc concentrates was proposed. The process can directly extract indium from removed copper solution by D2EHPA, and cancel the stage of removing iron in the traditional process because of using iron and part of zinc in the In-Zn concentrates for direct preparing high quality Mn-Zn soft magnetic ferrites. The technologies in the processes, such as leaching the neutral leached residues with high concentrated acid at high temperature, reduction ferric and removing copper, and extracting indium, were investigated. The results show that total recovery ratio of indium is increased from less than 70% in the traditional process to more than 95%. This process has the advantages of largely simplifying the procedure of indium extraction, zero draining off of iron residue and zero emitting of SO2. So this is a clean production process.
Key words:
indium; extraction; hot acid leaching; zinc; neutral leached residue;
1 IntroductionThe typically traditional process for extracting indium from In-Zn concentrates includes the following steps. Firstly, ferric is removed by the method of jarosite, and indium goes into jarosite residues[1-4]. In order to recovery indium from the jarosite residues, the further treatments of jarosite residues are needed. As a result, the recovery ratio of indium is low, and serious pollutions are produced, such as low concentrate of sulfur dioxide and large quantity of ferric oxide residues [5-12].
In our application, the hydrometallurgy of zinc is combined with the preparation of soft magnetic materials, and indium is directly extracted from ferrous suphate solution by D2EHPA[13-16]. Therefore, the flow sheet of hydrometallurgical extracting zinc and recovering indium is shortened and recovery ratios of valuable metals are greatly increased.
2 Raw materials and principle flow sheet
2.1 Raw materials
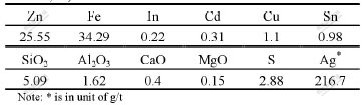
Raw material used in the experiments was neutral leached residues, which were prepared under general technical conditions by neutral leaching the calcine. The compositions of calcine and neutral leached residues are listed in Table 1 and Table 2, respectively, and the phase composition of zinc and iron in the neutral leached residues is listed in Table 3.
Table 1 Chemical composition of calcine (mass fraction, %)

Table 2 Chemical composition of neutral leached residue (mass fraction, %)
Chemical composition of zinc sulfide concentrates used as reduction agent is listed in Table 4. The content of Mn in pyrolusite, used as manganese resource and oxidation agent, is 38.65%.
Table 3 Phase composition of zinc and iron in neutral leached residue (mass fraction, %)
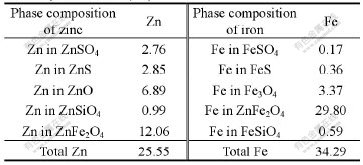
Table 4 Chemical composition of zinc sulfide concentrates (mass fraction%)
2.2 Principle flow sheet
Experimental principle flow sheet is shown in Fig.1.
3 Experimental
3.1 Experimental apparatus
The apparatus used in the first three procedures of reduction with ZnS concentrate, oxidation with pyrolusite, removing copper with iron powder, contained a temperature adjustable magnetic KHCB-3 stirrer, which has a deviation of 1.5 ℃, a 500 mL-flask for conditional experiments and a 5 000 mL-flask for comprehensive experiments. The apparatus used in the experiments of indium extraction included five funnels to separate water phase from organic phase, and a general vibrator.
3.2 Experimental methods
A series of experiments on reduction and oxidation leaching by concentrated acid at high temperature were carried out by the single factor test method with a scale of 100 g of neutral leached residues per time. The experiments of removing copper with a scale of 4 L per time were carried out for 45 min at 323-333 K, and iron powder used as a reductive agent with an addition amount of 2.1 times of the theoretic amount for reducing Fe3+ to Fe2+ and cementing Cu2+ and Cd2+ to metals. The method of indium extraction experiments was the same as the general extractive experiments.
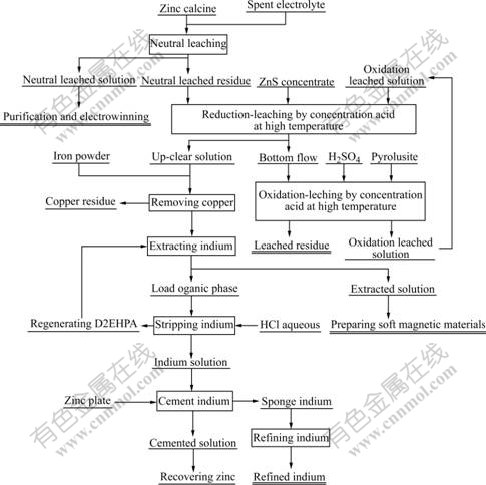
Fig.1 Principle flow sheet of extracting indium from In-Zn concentrates
3.3 Analysis methods
Iron, zinc, and indium in the experimental samples were analyzed by means of the oxidation-reduction titration by pyrochromic potassium, complex titration with EDTA, and atomic absorption spectrum, respectively.
4 Results and discussion
4.1 Reduction and oxidation leaching by concentrated acid at high temperature
4.1.1 Reduction-leaching factor
Using zinc sulfide concentrate from Zhuzhou Smelter as reduction agent, effects of time, granule size and its addition methods on leaching ratio are investigated.
1) Effect of time
A serial of experiments are conducted to investigate the effect of time on the reductive leaching under the conditions as follows. The leaching temperature is 95 ℃, the mass ratio of liquid to solid is 8, and the addition amount of sulphuric acid is 1.692 g per gram neutral leached residue. The results are shown in Fig.2.

Fig.2 Effect of time on reductive leaching: 1, 2, 3 Leaching ratio of indium, iron and zinc respectively; 4 Reduction ratio of ferric; 5 Residue ratio
It is shown in Fig.2 that the leaching ratio of indium, iron, zinc and the reduction rate of ferric are all increased, but residue ratio is decreased with prolonging the time. Reduction ratio of Fe3+ is the highest after leaching for 5 h. So the best leaching time is 5 h.
2) Effect of reductive agent addition amount
In the case of leaching for 5 h and the other given conditions are as same as described above in 1), the effect of addition amount of the reductive agent on reduction-leaching is shown in Fig.3.
In Fig.3, it is shown that the leaching ratio of indium, iron, and especially zinc is decreased, but the reduction ratio of Fe3+ is increased with the addition amount of reductive agent increasing. The leaching ratio of zinc is decreased and the residue ratio is increased, which indicates that it is difficult to leach iron and zinc from the zinc sulfide concentrate. The optimum addition amount of reduction agent is 1.03 times of theoretic amount for reducing Fe3+ to Fe2+.

Fig.3 Effect of reduction agent amounts on reductive leaching: 1, 2, 3 Leaching ratio of indium, iron and zinc respectively; 4 Reduction ratio of ferric; 5 Residue ratio
3) Effect of granule size of reduction agent
The addition amount of reduction agent is chosen as 1.38 times of theoretic amount for reducing Fe3+ to Fe2+, and the other conditions are maintained as described above in 2). The effect of the reduction agent granule size on reduction-leaching is shown in Fig.4.
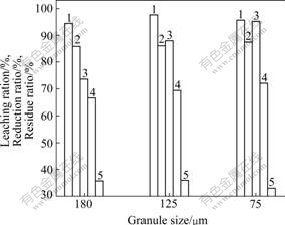
Fig.4 Effect of reduction agent granule size on reductive- leaching: 1,2,4 Leaching ratio of indium, iron and zinc, respectively; 3 Reduction ratio of ferric; 5 Residue ratio
It is shown in Fig.4 that the reduction ratio of Fe3+ is quickly increased. The leaching ratio of metals is also increased but the leaching ratio of zinc is not high. And the residue ratio is decreased with decreasing reduction agent granule size.
4) Effect of addition method of reduction agent
In the case of leaching for 3 h and the other given conditions as described above in 3), the effect of the addition method of reductive agent on reduction leaching is shown in Table 5.
From Table 5, it is shown that the effect of the addition method on leaching ratio of Fe and In is small. However, when the reductive agents are added by four times, the reduction ratio of Fe3+ and the leaching ratio of Zn are quickly decreased.
5) Comparative experiments of zinc sulfide concen- trates from different fields
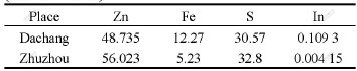
In the case of leaching for 5 h and the other given conditions as described above in 1), the comparative experiments on the reductive agent using zinc sulfide concentrates from different areas are carried out. The results are shown in Table 6.
Data in Table 6 show that the reduction efficiencies of zinc sulfide concentrates from different fields are much different. The reduction efficiency of zinc sulfide concentrate from Dachang is the best one, while that of zinc sulfide concentrate from Zhuzhou is the worst because of its complicated compositions.
4.1.2 Oxidation leaching condition
In order to increase leaching ratio of Zn and In and to prepare soft magnetic materials, the oxidation leaching with concentrated acid at high temperature is carried out by using pyrolusite as oxidation agent after the reduction-leaching, in which the zinc sulfide concentrate from Dachang is used as reduction agent under the optimum conditions. The effects of time and the initial acid concentration on oxidation leaching at 368 K are examined under the following conditions. The mass ratio of liquid to solid is 2.4, and the addition amounts of sulphuric acid and pyrolusite are 0.699 2 g and 0.26 g respectively for per gram of neutral leached residue.
1) Effects of time
The effects of time on oxidation leaching are shown in Fig.5.
It is shown in Fig.5 that the leaching ratios of In and Zn are very high, but the residue ratio is very low after leaching for 5 h.
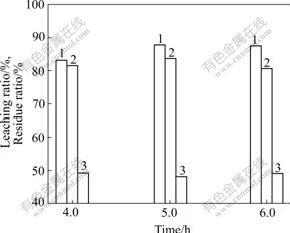
Fig.5 Effects of time on oxidation leaching: 1, 2 Leaching ratio of zinc and indium, respectively; 3 Residue ratio
2) Effects of initial acid concentration
The effects of the initial acid concentration on oxidation leaching are shown in Fig.6. From Fig.6, the leaching ratios of In and Zn are increased with increasing initial acid concentration, and the residue ratio of is decreased accordingly. The best result is obtained when the initial acid concentration is 250 g/L.
4.1.3 Comprehensive experiments
Based on the results of factor experiments, the reductive leaching experiments are carried out with the initial acid concentration of 225 g/L at 368 K for 5 h, and the adding amount of zinc sulfide concentrate from Dachang Smelter, which is added by four times, is 1.03 times of theoretical amount for reducing Fe3+ to Fe2+. 4 h later, the iron powders with 2%-3% of neutral leached residue mass are added. Similarly, when the initial acid concentration is higher than 250 g/L and the amount of pyrolusite is 0.25 g for per gram neutral leached residue, the oxidation leaching experiments are carried out at 368 K for 5 h. The comprehensive experiments are carried out circularly under the optimum conditions, and the results of the comprehensive experiments are listed in Tables 7-9.
Table 5 Effect of addition method of reductive agent on reduction leaching

Table 6 Effect of resource of zinc sulfide concentrates on reduction leaching


Fig.6 Effects of initial acid concentration on oxidation leaching: 1, 2 Leaching ratio of zinc and indium, respectively; 3 Residue ratio
The average leaching ratios of In, Zn, and Fe are 96.76%, 96.25%, and 86.59% respectively, and the reduction ratio of ferric is more than 94%.
4.2 Removing copper
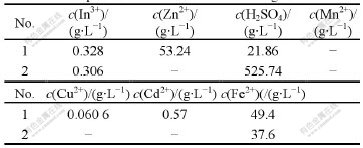
The compositions of filtrate of removed copper and copper residue are listed in Table 10 and Table 11, respectively. The technical targets of removing copper are listed in Table 12.
Data in Tables 10-12 indicate that the average separation efficiency of copper is 94.61% and the recovery ratio of indium is as high as 99.47%. In addition, the content of copper in copper residue is as high as 74.20%, which is beneficial to the recovery of copper.
4.3 Extracting indium
4.3.1 Solution for experiments
Two batches of solution of removed copper, the chemical compositions of which are listed in Table 13, are used in the experiments of extracting indium.
4.3.2 Experiments of extracting indium
According to Refs.[8,9], the optimum conditions of extracting indium by D2EHPA are as follows. The organic phase consists of 30% D2EHPA and 70% sulphonated kerosene, and the phase ratio is equal to 3, the time for vibration and setting are 5 min and 10 min respectively, and the acid concentration in solution phase is 20 g/L. The indium extraction is conducted at 298 K by three-stage counter-current process.
Table 7 Composition of recycle leaching solutions
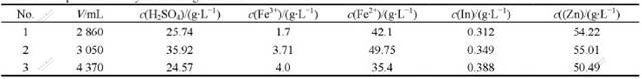
Table 8 Composition of recycle leaching residues
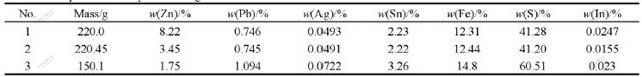
Table 9 Technical targets of recycle leaching experiments

Table 10 Composition of filtrate of removed copper

Table 11 Composition of copper residue
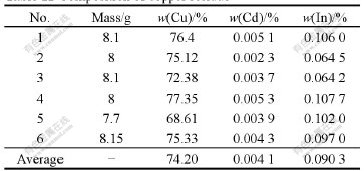
Table 12 Technical targets of removing copper
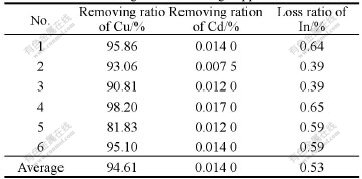
Table 13 Compositions of solution for extracting indium
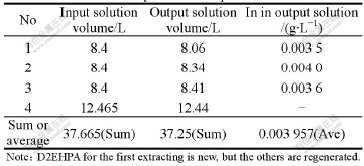
Under the above optimum conditions, the comprehensive experiments of extracting indium are carried out circularly by the method of simulated counter-current extraction. The results of using the first batch of solution are listed in Table 14.
As shown in Table 14, it can be seen that the extraction ratio of indium with the first solution is as high as 98.92%. The content of indium in the second output solution is 0.0024 g/L, so the extraction ratio of indium is 99.23%. In conclusion, the average extraction ratio of indium is 99.075%.
Table 14 Results of comprehensive experiment on first solution
4.3.3 Experiments on indium striping
The experiments on stripping indium are carried out under the general stripping conditions as follows. The ratio of organic phase to solution is equal to 15, time for vibration and setting is 5 min, and a HCl solution of 5 mol/L is used as stripping agent of for three-stage striping at 298 K. And the acid washing experiments are conducted under the following conditions. The ratio of organic phase to solution is equal to 4, time for vibration and setting is 5 min, and a H2SO4 solution of 150 g/L is used as washing acid at 298 K. As a result, the volumes of the first and the second stripping solution are 805 mL and 305 mL, respectively, and the contents of indium in stripping solution are 13.958 g/L and 10.97 g/L respectively. So the stripping ratios are both more than 99%.
4.3.4 Experiments on cementing indium
Indium is cemented by zinc plate from the striping solution at room temperature and spongy indium is obtained. The result shows the cemented ratio of indium is more than 99%.
5 Conclusions
1) Compared with traditional hydrometallurgical process of zinc,the new process for extracting indium is short and simple because of cancellation of iron removing process.
2) Under the optimum conditions, the leaching ratios of In, Zn, and Fe are 96.76%, 96.25%, and 86% respectively, and the reduction ratio of Fe3+ is higher than 94% in circular reduction-oxidation leaching with concentrated acid at high temperature.
3) The cemented copper ion with iron powder is feasible, the separation ratio is 94.61% and the content of copper in copper residue is 74.20%.
4) Indium is directly extracted from copper removing solution, and the direct and total recovery ratios of indium are more than 93% and 95%, respectively.
References[1] MEI Guang-gui, WANG De-run, ZHOU Jin-yuan, WANG Hui. The Hydrometallurgy of Zinc [M]. Changsha: Central South University Press, 2001. 32-33, 140-143.
[2] MA Rong-jun. The problem of removing iron in hydrometallurgy of zinc [J]. Hunan Non-ferrous Metal, 1993(3): 161-164.
[3] SEYER S, CHEN T T, DUTRIZAC J E. Addressing iron disposal in the zinc industry [J]. JOM, 2001, 53(12): 32.
[4] NING Shun-ming, CHEN Zhi-fei. Recovering indium from goethite residues [J]. Trans Nonferrous Met Soc China, 1997, 7(3): 56-58.
[5] TANG Mo-tang. The recovery of metals and products deep processing being used the waste residues in the hydrometallurgy of zinc as metals sources [A]. Chinese Academy of Engineering. Academician Conference Symposia on Technical Innovation of Guangxi Nonferrous Metal Industry [C]. Liuzhou: 2002. 168.
[6] HIDEKI A. Recovery of gallium and indium from zinc refinery by-product [J]. Nippon Kogyo Kaishi, 1982, 98(1133): 561-565.
[7] LU Jun-le, ZHONG Zhu-qian, WU Tie-hui. Theoretical analysis of goethite iron elimination process by reduction with zinc sulfite in zinc hydrometallurgy [A]. YAZAWA A. Zinc ′85: Proceedings of International Symposium on Extractive Metallurgy of Zinc [C]. Tokyo: 1985. 659-674.
[8] HALSALL P. Indium-extration from lead, zinc and tin circuits [J]. Transactions of the Institution of Mining and Metallurgy, Section C, 1988, 97: 93-101.
[9] ZHOU Tai-li, ZHONG Xiang, ZHENG Long-gao. Recovering In, Ge and Ga from zinc residues [J]. JOM, 1989, 41(6): 36-40.
[10] MANTELL C L. Electrometallurgy in aqueous solutions [A]. DETURIC C. Met Soc AIME Proc Extractive Metallurgy Div Symp on Electrometallurgy [C]. Cleveland: AIME, 1969. 52-82.
[11] FORTES M C B, MARTINS A H, BENEDETTO J A.. Indium recovery from acidic aqueous solutions by solvent extraction with D2EHPA: A statistical approach to the experimental design [J]. Brazilian Journal of Chemical Engineering, 2003, 20(2): 121-128.
[12] FORTES M C B, MARTINS A H, BENEDETTO J S. Indium absorption onto ion exchange polymeric resins [J]. Minerals Engineering, 2003, 16(17): 659-663.
[13] TANG Mo-tang, LI Shi-qing, YANG Sheng-hai, TANG Chao-bo, HE Jing, PENG Chang-hong, YAO Wei-yi, LU jun-le, ZHANG Bao-ping, XIA Zhi-hua. The hydrometallurgy of zinc without residue [P]. CN03118199.2, 2003.3.
[14] TANG Mo-tang, LI Shi-qing, YANG Sheng-hai, TANG Chao-bo, HE Jing, PENG Chang-hong, YAO Wei-yi. The hydrometallurgy of zinc and extracting indium without iron residues [J]. Nonferrous Metals(Extractive Metallurgy), 2004(6): 27-29, 34.
[15] MA Rong-jun. Recovery of indium by extraction in zinc hydrometallurgy by use of leaching by high concentrated acid at high temperature and removing iron by the goethite method [J]. Hydrometallurgy, 1997, 62(2): 58-61.
[16] LI Shi-qing, LIU Wei-feng, TANG Mo-tang, HE Jing, LU Jun-le. Extracting indium from a reduction solution added manganese in the hydrometallurgical process of zinc without iron residues [J]. Journal of Jishou University (Natural Sciences Edition), 2004, 25(4): 14-18.
(Edited by YANG Bing)
Foundation item: Project(2004AA649080) supported by the National Basic Research and Development Program of China; Projects(50234010, 50444020) supported by the National Natural Science Foundation of China
Corresponding author: LI Shi-qing; Tel: +86-731-8830470; E-mail: hetyy@mail.csu.edu.cn


