文章编号:1004-0609(2011)09-2146-05
Al掺杂对Li4Ti5O12结构及性能的影响
熊训辉, 王志兴, 伍 凌, 李新海, 吴飞翔, 郭华军, 彭文杰
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:
以金红石型TiO2、Li2CO3和Al2O3为原料,采用高温固相法制备锂离子电池负极材料Li4Ti5O12和Li4AlxTi5-xO12(x=0, 0.025, 0.05, 0.1, 0.2, 0.4)。利用X射线衍射仪、扫描电镜、半电池充放电测试和交流阻抗测试研究材料的物相、结构、形貌以及电化学性能。结果表明:Al掺杂不会改变Li4AlxTi5-xO12的尖晶石结构,但会导致材料颗粒尺寸增大;适当Al掺杂后,材料的循环稳定性和极化性能得到改善,充放电比容量和可逆比容量不同程度降低;Li4Al0.025Ti4.975O12具有最优的电化学性能,0.1C倍率下首次充电比容量达到156.7 mA·h/g。
关键词:
中图分类号:TM912.9 文献标志码:A
Effect of Al-doping on structure and
eletrochemical performance of Li4Ti5O12
XIONG Xun-hui, WANG Zhi-xing, WU Ling, LI Xin-hai , WU Fei-xiang, GUO Hua-jun, PENG Wen-jie
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Anode materials Li4Ti5O12 and Li4AlxTi5-xO12(x=0, 0.025, 0.05, 0.1, 0.2, 0.4) were prepared by solid-state reaction using rutile TiO2 and Li2CO3 as the reactants. The crystalline structure, morphology, composition and electrochemical properties of the as-prepared materials were characterized by XRD, SEM and half cell charge-discharge testing and AC impedance. The results show that all of the prepared Li4AlxTi5-xO12 keep the structure of spinel, however, the particle size of samples with Al-doping increases in a certain extent and the appropriate amount of Al doping in Li4Ti5O12 leads to obvious decrease in initial and the reversible specific capacity, but improves the cycling stability and electrochemical polarization. In this study, Li4Al0.025Ti4.975O12 shows the best electrochemical performance and delivers the first discharge specific capacity of 156.7mA·h/g at 0.1 C.
Key words: lithium titanate; Li-ion battery; anode material; Al-doping
随着锂离子电池逐渐用于混合动力和纯电动汽车上,对电池材料的安全性、使用寿命和倍率性能提出了更高的要求。与炭类负极材料相比,尖晶石型Li4Ti5O12是零应变材料,循环性能好、充放电电压平台平稳、安全性能好且容易制备,是很有前途的动力型锂离子电池负极材料[1-3]。通常认为锐钛型TiO2是Li+嵌入的活性主体,而金红石型TiO2几乎不能嵌入Li+[4],所以研究者主要用锐钛型TiO2和锂源按化学计量比在800~1 000 ℃内烧结合成Li4Ti5O12材料。
Li4Ti5O12的诸多优点使其有希望成为替代炭类材料作为一种锂离子动力型的负极材料,但其电子导电性影响了其倍率性能的发挥[5],阻碍了它的发展和商业化。研究者主要通过细化晶粒[6]、包覆导电性物质[7-9]和掺杂金属阳离子[10-14]等途径提高Li4Ti5O12的倍率性能。已报道的掺杂离子如Mg2+、Fe3+、F-、Mn2+、V3+等对Li4Ti5O12材料的电化学性能有一定程度的改善,但仍存在不足。TIAN等[15]发现Nb5+掺杂可提高材料的电化学性能,但其成本较高、操作工艺复杂,不易实现规模化生产。因此,寻找一种更适合的掺杂离子并通过简单有效的方法即能全面提高材料的性能是很有必要深入研究的。Al-O八面体非常稳定且Al的质量小,本文作者将以来源广、成本低的金红石型TiO2为原料合成Li4Ti5O12材料,研究Al3+掺杂对高温固相法合成的Li4Ti5O12结构和性能的影响。
1 实验
按Li4AlxTi5-xO12(x=0, 0.025, 0.05, 0.1, 0.2, 0.4)化学计量比(Li盐过量4%以弥补高温下的挥发)分别称取Li2CO3、Al2O3和金红石型TiO2,以无水乙醇作为分散剂,室温下球磨4 h,混合物于80 ℃烘干后置入程序控温管式炉,在空气气氛中加热至850 ℃,保温16 h后随炉冷却,玛瑙中手工研细10 min得到样品,分别标记为0Al、0.025 Al、0.05Al、0.1Al、0.2Al、0.4Al。
采用X射线衍射仪(Rigaku公司生产,日本)对合成的材料进行物相分析,以CuKα靶作为辐射源,电压为40 kV,电流为50 mA,步宽为0.02°,扫面速度为2(°)/min,衍射角的扫描范围为10°~85°。用JEOL公司生产的JSM-5600LV型扫描电子显微镜观察材料形貌。
将材料制成CR2025型扣式电池进行充放电测试。采用涂膜法制备电极,以N-甲基-2-吡咯烷酮为溶剂,按质量比8 ? l ? l分别称取活性物质、乙炔黑和聚偏二氟乙烯 (PVDF),混合均匀后,涂在预处理过的铝箔上,120℃干燥6 h后制成直径为14 mm的正极片。以金属锂片作为负极,微孔聚丙烯膜(Celgard2400, USA)为隔膜,以l.0 mol/L 六氟磷酸锂/碳酸乙烯酯、碳酸二甲酯和碳酸甲乙酯(体积比为l ? l ? l)为电解液,组装成扣式电池。用新威BTS-5V/1mA电池测试系统室温下在不同倍率进行充放电循环测试,电压范围为1.0~2.5 V。交流阻抗测试在美国CHI660电化学工作站上完成,测试频率为0.01 Hz~100 kHz。
2 结果与讨论
图1所示不同掺铝量合成样品的XRD谱,对照尖晶石Li4Ti5O12的标准图谱可知,所有样品的特征峰与尖晶石型Li4Ti5O12的标准图谱(49-0207)基本吻合,均为尖晶石结构。掺杂量x=0.025~0.2时, Al3+全部进入Li4Ti5O12的晶格(见图1(a)),形成良好的固溶体;掺杂量x=0.4时,样品中存在Al2O3杂质,说明掺杂量不宜过大。与未掺杂样品相比,掺杂样品在2θ约为27.4°处均出现TiO2杂质峰。这是因为Al3+取代Li4Ti5O12(Fd![]() m)16d位的Ti4+时[16],为保持电子平衡,产生一定量的Ti4+/Ti3+混合价态,在空气气氛煅烧时Ti3+被氧化成Ti4+,从电子平衡角度考虑,Ti元素过量,所以导致TiO2的产生[13]。
m)16d位的Ti4+时[16],为保持电子平衡,产生一定量的Ti4+/Ti3+混合价态,在空气气氛煅烧时Ti3+被氧化成Ti4+,从电子平衡角度考虑,Ti元素过量,所以导致TiO2的产生[13]。
图1 (b)所示为不同掺铝量合成样品的(111)峰放大图。由图1(b)可见,掺杂样品的特征峰强度随掺Al3+量的增加而减弱,其原因是Al3+半径和电负性与Ti4+的有所不同,材料发生晶格畸变 [17-18]。掺杂样品的(111)峰逐渐向2θ角度增大方向偏移,可知掺杂样品的晶胞常数逐渐减小,这与经FULLPROF程序拟合计算得到的各样品的晶胞常数(见表1)一致,其原因归结于Al3+的半径(0.053 nm)比Ti4+的半径(0.061 nm)小。
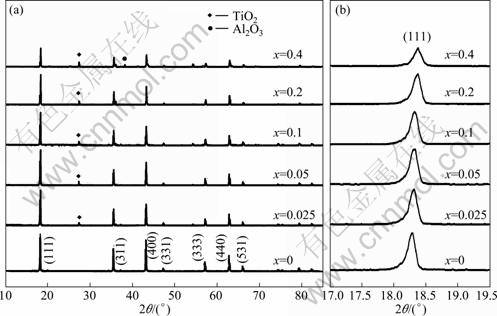
图1 不同掺铝量样品Li4AlxTi5-xO12的XRD谱
Fig.1 XRD patterns of Li4AlxTi5-xO12 doped with different amounts of Al
表1 样品 Li4AlxTi5-xO12(x=0, 0.025, 0.05, 0.1, 0.2, 0.4)的晶胞常数
Table 1 Lattice parameters of Li4AlxTi5-xO12(x=0, 0.025, 0.05, 0.1, 0.2, 0.4) samples

图2所示为不同掺铝量合成样品的SEM像。从图2可看出,样品颗粒尺寸随着掺铝量的增大而增大,纯相样品的颗粒尺寸约250 nm,掺铝样品颗粒尺寸都在1 μm以上, Li4Al0.4Ti4.6O12样品颗粒已熔合形成致密的固体。其原因是掺铝导致Li4Ti5O12材料熔点有所降低,与ZHAO等[13]报道的相同。
图3所示为不同掺铝量合成样品的首次充放电曲线和循环性能曲线。从图3(a)可以看出,未掺杂材料0.1 C倍率下首次充电比容量为161.6 mA·h/g,与锐钛型TiO2为钛源合成材料的性能相当[19-20],说明对于高温固相反应而言,钛源TiO2的形态对合成材料的性能影响不大。与未掺杂样品比较,所有掺Al3+样品首次充放电比容量均不同程度减小,样品0.025Al的电化学性能最优,首次充电比容量为156.7 mA·h/g,2 C倍率下比容量仅为61.9 mA·h/g, 20次循环后容量保持率为98.9%。其原因是Al3+掺杂导致Li4Ti5O12材料颗粒尺寸变大,Li+的迁移路径增长,扩散阻力增大,导致首次充放电容量降低和倍率性能变差[21]。从图3(a)还可知,样品0.025Al、0.05Al 、0.1Al和0.2Al的极化性能有所改善,样品0.4Al的极化反而增大。这是因为掺Al3+提高材料的电子导电性[22]的同时会引起材料晶胞收缩,导致Li+脱嵌困难,且掺杂在晶格中的Al3+会阻碍Li+扩散。当掺铝量低时,电子导电性占优势,所以样品极化减小;当掺铝量较高时,Li+扩散速度降低更为明显。

图2 不同掺铝量样品Li4AlxTi5-xO12的SEM像
Fig.2 SEM images of Li4AlxTi5-xO12 doped with different amounts of Al: (a) x=0; (b) x=0.025; (c) x=0.05; (d) x=0.1; (e) x=0.2; (f) x=0.4
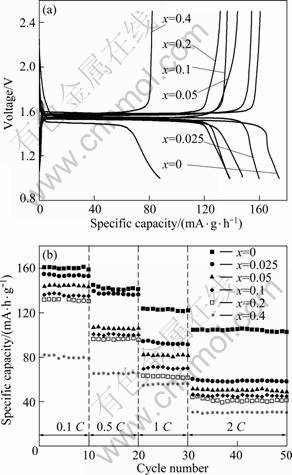
图3 样品Li4AlxTi5-xO12的首次充放电曲线与循环曲线
Fig.3 Initial charge-discharge curves (a) and cycle performances (b) of Li4Ti5-xAlxO12 samples
从图3(b)中看,所有掺杂样品因颗粒粗大导致高倍率下的充放电比容量迅速降低;但由于Al-O八面体很稳定,使样品循环稳定性能很好,容量保持率均在98%以上。
图4所示为不同掺杂铝量样品Li4AlxTi5-xO12的交流阻抗谱图。图中高中频区的半圆反映的是电荷转移过程的阻抗以及电极和电解液间的界面容抗;在低频区的斜线反映的则是锂离子在固态活性物质中扩散引起的Warburg阻抗[23],其特点是斜率越大,阻抗值 越小[24]。
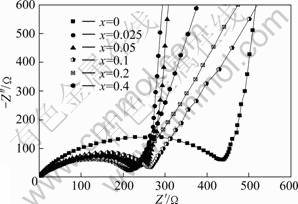
图4 不同掺铝量样品Li4Ti5-xAlxO12的交流阻抗图
Fig.4 AC impedance spectroscopies of Li4AlxTi5-xO12 doped with different amounts of Al
从图4可知,掺杂样品在高中频区的半圆与实轴截距在220~260 Ω之间,比未掺杂样品的直径明显减小,掺杂样品的界面反应阻抗有所降低,电子导电性有所提高。在低频区域,样品0.025Al和0.05Al的直线斜率与样品0Al的相近,样品0.1Al、0.2Al和0.4Al的直线斜率均要小于样品0Al的,说明掺杂Al3+进入晶格后不同程度地阻碍锂离子的扩散。
Al3+掺杂提高材料的导电性可归于空穴导电机 理[23],Al原子的价电子结构为3s23p1,以其取代Li4Ti5O12中的Ti4+离子时缺少一个电子,因此会有一个单电子键。此单电子键必将从Ti—O键的获得电子,导致在Ti—O键的位置显示过剩的正电荷,即能带理论中的空穴导电,而Al3+附近也将呈现一定的负电荷过剩,形成一个位置固定的负空间电荷。
3 结论
1) 分别以金红石型TiO2和锐钛矿TiO2为钛源合成的Li4Ti5O12电化学性能相近,以金红石型TiO2为钛源可以降低该材料的成本。
2) Al3+掺杂不会改变Li4Ti5O12材料尖晶石结构,但空气气氛条件下合成Li4Ti5O12材料时会引入杂质相TiO2,并使Li4Ti5O12材料颗粒明显粗大。Al3+掺杂增强Li4Ti5O12材料导电性的同时也会阻碍锂离子的扩散,二者对Li4Ti5O12的电化学性能有很大影响。
3) Li4Al0.025Ti4.975O12在实验条件下具有最优的 电化学性能,0.1C倍率下首次充电比容量为 156.7 mA·h/g,2 C倍率下20次循环后容量保持率为98.9%。
REFERENCES
[1] PANERO S, SATOLLI D, SALOMON M, SCROSATI B. A new type of lithium-ion cell based on the Li4Ti5O12/ Li2Co0.4Fe0.4Mn3.2O8 high-voltage, electrode combination [J]. Electrochemistry Communication, 2000, 2(11): 810-813.
[2] OHZUKU T, UEDA A, YAMAMOTO N. Zero-strain insertion material of Li4Ti5O12 for rechargeble lithium cells[J]. Journal of the Electrochemical Society, 1995, 142(5): 1431-1435.
[3] ZAGHIB K, SIMONEAU M, ARMAND M, GAUTHIER M. Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries[J]. Journal of Power Source, 1999, 81: 300-305.
[4] STASHANS A, LUNELL S, BERGSTROM R, HAGFELDT A. Theoretical study of lithium intercalation in rutile and anatase[J]. Physical Review, 1996, 53(1): 145-149.
[5] OUYANG C Y, ZHONG Z Y, LEI M S. Ab initio studies of structural and electronic properties of Li4Ti5O12 spinel[J]. Electrochemistry Communication, 2007, 9(5): 1107-1112.
[6] GUERFI A, SEVIGNY S, LAGACE M, HOVINGTON M, KINOSHITA K, ZAGHIB K. Nano-particle Li4Ti5O12 spinel as electrode for electrochemical generators [J]. Journal of Power Source, 2003, 88: 119-121.
[7] HE Ze-qiang, XIONG Li-zhi, CHEN Shang, WU Xian-ming, LIU Wen-ping, HUANG Ke-long. In situ polymerization preparation and characterization of Li4Ti5O12 polyaniline anode material[J]. The Chinese Journal of Nonferrous Metals, 2010, 20: 262-266.
[8] DOMINKO R, GABERSCEK M, BELE M. MIHAILOVIC D. Carbon nano-coatings on active materials for Li-ion batteries[J]. Journal of the European Ceramic Society, 2007, 27(2/3): 909-913.
[9] HUANG S H, WEN Z Y, ZHANG J C, YANG X L. Improving the electrochemical performance of Li4Ti5O12/Ag composite by an electroless deposition method [J]. Journal of Electrochimica Acta, 2007, 52: 3704-3708.
[10] JI S Z, ZHANG J Y, WANG W W, HUANG Y, FENG Z R, ZHANG Z T, TANG Z L. Preparation and effects of Mg-doping on the electrochemical properties of spinel Li4Ti5O12 as anode material for lithium ion battery [J]. Journal of Materials Chemistry and Physics, 2010.
[11] MUKAI K, ARIYOSHI K, OHZUKU T. Comparative study of Li[CrTi]O4, Li[Li1/3Ti5/3]O4 and Li1/2Fe1/2[Li1/2Fe1/2Ti]O4 in non-aqueous lithium cells[J]. Journal of Power Sources, 2005, 146: 213-216.
[12] HUANG S H, WEN Z Y, GU Z H. Preparation and cycling performance of Al3+ and F- co-substituted compounds Li4AlxTi5-xFyO12-y [J]. Journal of Electrochimica Acta, 2005, 50: 4057-4062.
[13] KUBIAK P, GARCIA A, WOMES M, ALDON L, OLIVIER-FOURCADE J, LIPPENS P E, JUMAS J C. Phase transition in the spinel Li4Ti5O12 induced by lithium insertion influence of the substitutions Ti/V, Ti/Mn, Ti/Fe [J]. Journal of Power Sources, 2003, 119: 626-630.
[14] ZHAO H L, Li Y, ZHU Z M, LIN J, TIAN Z H, Wang R L. Structural and electrochemical characteristics of Li4-xAlxTi5O12 as anode material for lithium-ion batteries[J]. Journal of Electrochimica Acta, 2008, 53: 7079-7083.
[15] TIAN Bing-bing, XIANG Hong-fa, WANG Hai-hui. Niobium doped lithium titanate as a high rate anode material for Li-ion batteries[J]. Journal of Electrochimica Acta, 2010, 55(19): 5453-5458.
[16] WU Xiao-mei, ZONG Xiang-fu, YANG Qing-he, JIN Zhong-kao, WU Hao-qing. Electrochemical studies of substituted spinel LiAlyMn2-yO4-zFz for lithium secondary batteries[J]. Journal of Fluorine Chemistry, 2001, 107: 39-44.
[17] ROBERTSON A D, TUKAMOTO H, IRVINEA J T S. Li1+xFe1-3xTi1+2xO4 (0.0≤x≤0.33) based spinels: Possible negative electrode materials for future li-ion batteries[J]. Journal of The Electrochemical Society, 1999, 146 (11): 3958-3962.
[18] REALE P, PANERO S, RONCI F, ROSSI A V, SCROSATI B. Iron-substituted lithium titanium spinels: Structural and electrochemical characterization[J]. Chemistry Material, 2003, 15: 3437-3442.
[19] 姚经文, 吴 锋. 锂离子电池负极材料Li4Ti5O12的合成及电化学性能[J]. 功能材料, 2006, 37(11): 1752-1754.
YAO Jing-wen, WU Feng. Preparation and characterization of Li4Ti5O12 anode material for lithium secondary batteries[J]. Journal of Functional Materials, 2006, 37(11): 1752-1754.
[20] 阮艳莉, 唐致远, 彭庆文. 尖晶石Li4Ti5O12电池材料的合成与电化学性能研究[J]. 无机材料学报, 2006, 21(4): 873-879.
RUAN Yan-li, TANG Zhi-yuan, PENG Qing-wen. Synthesis and eletrochemical performance of spinel Li4Ti5O12 eletrode material[J]. Journal of Inorganic Materials, 2006, 21(4): 873-879.
[21] VENKATESWARLU M, CHEN C H, DO J S, LIN C W, CHOU T C, HWANG B J. Electrochemical properties of nano-sized Li4Ti5O12 powders synthesized by a sol-gel process and characterized by X-ray absorption spectroscopy[J]. Journal of Power Sources, 2005, 146(1/2): 204-206.
[22] 方俊鑫, 殷之文. 电介质物理学[M]. 北京: 科学出版社, 1989: 245-247.
FANG Jun-xin, YIN Zhi-wen. The dielectric physics[M]. Beijing: Science Press, 1989: 245-247.
[23] 曹楚南, 张鉴清. 电化学阻抗谱导论[M]. 北京: 科学出版社, 2002: 20-36.
CAO Chu-nan, ZHANG Jian-qing. The introduction of AC impedance spectra [M]. Beijing: Science Press, 2002: 20-36.
[24] RAISTRICK I D, HO C, HUGGINS R A. Lithium ion conduction in Li5A1O4, Li5GaO4 and Li6ZnO4[J]. Materials Research Bulletin, 1976, 11(8): 953-957.
(编辑 何学锋)
基金项目:国家重点基础研究发展计划资助项目(2007CB613607)
收稿日期:2010-09-30;修订日期:2011-01-03
通信作者:王志兴,教授,博士;电话:0731-88836633;E-mail: xxhui19881118@163.com


