J. Cent. South Univ. (2017) 24: 1953-1958
DOI: https://doi.org/10.1007/s11771-017-3603-9

Activities of laccase produced by a strains Penicillium simplicissimum induced by chemical agentia and UV radiation
LI Xue(李雪)1, LI Fei(李飞)2, LAI Cui(赖萃)3, HUANG Jin-hui(黄瑾辉)3, PANG Ya(庞娅)1,LUO Kun(罗琨)1, LIAO Xing-sheng(廖兴盛)1
1. Department of Biological and Environmental Engineering, Changsha University, Changsha 410003, China;
2. School of Information and Safety Engineering, Zhongnan University of Economics and Law, Wuhan 430073, China;
3. College of Environmental Science and Engineering, Hunan University, Changsha 410082, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract:
Penicillium simplicissimum was cultured and preserved on the potato dextrose agar (PDA) medium. PDA-RBBR (Remazal Brilliant Blue R) medium was used for the screening of the strains, which is able to produce enzymes. After the mutation process in Penicillium simplicissimum induced by chemical reagent and ultraviolet radiation, a high laccase-producing strains Penicillium simplicissimum was obtained. When 5 mL diethyl sulfate (2%) was mixed along with 5 mL spore suspension for 30 min, chemical mutagenesis reached its best condition. And the optimum conditions of UV mutagenesis were that spore suspension was irradiated for 4 min under 15 W UV lamp at a distance of 30 cm. The highest activity of C5E4 strains was 4.80 U/g over 18% higher than the maximum laccase activity of original microorganism. Five generations of the mutant strains were cultured, and the laccase activity of the strains was measured. The result showed that C5E4 strains can product laccase of the five subcultures stably.
Key words:
Penicillium simplicissimum; laccase; chemical mutation; UV mutation;
1 Introduction
Laccase (benzendiol: oxygen oxidoreductase, EC 1.10.3.2), a kind of polyphenol oxidase, was firstly discovered in the Japanese tree Rhusvernicifera. It belongs to the group of multi-copper blue oxidases, and it is widely found in higher plants, insects, and bacteria [1, 2], especially in white-rot fungi [3, 4]. Laccase has a wide range of substrates such as multiple phenolic compounds, aminecompounds and their derivatives. It was also found that laccase can catalyze the oxidation of various phenolic and some non-phenolic substrates in the presence of mediator [5-7]. Thus, laccase plays an important role in the ligninbiotreatment, removing phenolic contaminants, degrading pesticides, decoloring dye and textile wastewater due to its broad substrate specificity, and increasing laccase activity is the key to improve the treatment effect [8-11].
In recent years, many studies have focused on breeding new laccase-producing strain and how to enhance their potential production capacity of laccase [7, 12]. Penicillium simplicissimum has been reported to degrade lignin mainly because of the catalysis of laccase and lignin peroxidase (LiP) [13, 14]. At present, the studies of Penicillium simplicissimum mainly focus on the determination of lignin degradation [15, 16], enhancement of the capabilities of generating laccase [17, 18], adsorption of heavy metal by fixed Penicillium simplicissimum [17, 19], biodegradation of pyrene [20, 21], degradation of heavy oil [22] and so on. Based on our previous studies [23-25], it was illustrated that the advantage soil fungi Penicillium simplicissimum had greatly potential economic value due to its good degradation of the toxic organics including phenol, aniline and onion quinine. Therefore, it is of significance to further improve the enzyme production ability of Penicillium simplicissimum for industrialized application.
In this study, the original microorganism of Penicillium simplicissimum was carried out chemical mutagenesis and UV mutagenesis treatment, respectively. And then through screening and rescreening, a stable mutant Penicillium simplicissimum of high laccase production was expected to be obtained.
2 Materials and methods
2.1 Bacterial strains
Penicillium simplicissimum was isolated from the soil of Yuelu mountain woodland in Changsha, China.
2.2 Activation and preservation of strains
Penicillium simplicissimum which had been preserved on slant plant at 4℃ was put into 30 °C incubator for 1 h for activation. In sterile conditions, Penicillium simplicissimum was inoculated on potato dextrose agar (PDA) plates (20 g glucose, 15 g agar,200 g potato, and 1000 mL distilled water) and cultured at 30 °C for several days.
2.3 Preparation of spore suspension
The spores were scraped by an aseptic cotton swab which was wet by sterile distilled water, and rubbed against the wall of biological tube gently in order that spores dissolved in sterile water until the concentration of the spore solution reached 107 mL-1.
2.4 Chemical mutagenesis
5 mL diethyl sulfate (2%) was added into 100 mL conical flasks which contains 5 mL spore suspension and 5 mL sterilized anaerobic phosphate buffer. And then 0.5 mL 2% sodium thiosulfate was added into the flasks to terminate the reaction after the flacks were oscillated for 15, 30, 45, 60 min, respectively. After this, 100 μL dilute mixture solution was pipetted and coated on PDA plate. And the plates were put in a constant temp incubator upside down at 30 °C. In order to obtain a high positive mutation, we observed the effect of mutation and calculated mortality to choose the dose which makes the lethal mutation rate become 70%-80%, as the control of DES was replaced by sterile distilled water.
2.5 UV mutagenesis
5 mL spore suspension of the higher laccase activity strains which was selected from the above chemical mutation was pipetted into diameter 90 mm sterile Petri dishes. And then the Petri dishes with magnetic stirring were placed under the 15 W UV lamp which had been irradiated stably for 30 min to be irradiated for 0, 1, 2, 3, 4, 5 min, respectively. After this, 100 μL above solution which had been diluted was pipetted and coated on PDA plates after they kept in dark place about 30 min. And the plates were put in a 30℃ incubator upside down. In order to obtain a high positive mutation, the effect of mutation and calculated mortality were observed to choose the right irradiate time which makes the lethal mutation rate become 70%-80%.
2.6 Screening mutant strains
The plate colonies of death rate 70%-80% were selected to inoculate on PDA-RBBR preliminary screening medium (5.0 mg/mL sterilized RBBR solution was added into sterilized PDA medium which had been cooling to 60℃ for that the concentration of RBBR in the PDA-RBBR plate is 625 mg/mL) and the colonies which made the PDA-RBBR screening plate fade better was selected to culture in solid fermentation culturing medium for the determination of enzyme activity.
2.7 Rescreening mutant strains
2.7.1 Solid state fermentation system
The conical flasks (500 mL) containing 30 g rice straw power and 90 mL liquid produced enzyme medium(NH4Cl 2.0 g, MgSO4·7H2O 0.5 g , KH2PO41.0 g, Na2HPO4·12H2O 0.504 g, MnSO4·H2O 0.039 g, CuSO4·5H2O 0.007 g, FeSO4·7H2O 0.007 g, and distilled water 1000 mL) were autoclaved at 105 °C for 30 min. After natural cooling, 5 mL spores suspension of the selected strains and the original microorganism were inoculated in the fermentation flasks. The flasks were incubated in a constant temperature incubator at 30 °C and each medium was adequately mixed with a sterilized glass stick under sterile conditions every day.
2.7.2 Preparation of crude enzyme
During the process of fermentation, 20 mL 50 mmol/L sodium tartrate buffer solution (pH=4.5) and (2±0.05) g of fermentation solid which was sampled from the fermentation flasks aseptically every 2 d were added to 100 mL flasks separately. At room temperature, the flasks were put on a 200 r/min rotary shaker for 1 h for oscillation and extraction. After this, the mixtures were transferred into 50 mL centrifuge tubes and centrifuged at 6000 r/min for 15 min-1. And then the supernatant was filtered through 0.45 μm pore size membrane. The filtrate was analyzed for activities of laccase.
2.7.3 Enzyme assays
With ABTS method, enzyme activity was expressed as units (U) which was defined as 1 mm of ABTS oxidized per min under the condition of pH 4.0 and 25 °C. In the experiment, ABTS as the substrate, the total volume of the reaction was 3 mL. 0.5 mL 5 mmol/L ABTS and 0.5 mL crude enzyme solution were added into 4 mL cuvette which contains 2.0 mL 0.1 mol/L citric acid-sodium citrate buffer (pH 5.0). Equal volume of buffer instead of crude extract was regarded as the control group. The solution was mixed and the change in absorbance was monitored at 420 nm, [ε420=36000 (mol/L)·cm] on a UV-2250 UV–vis spectrophotometer for 3 min. Each of samples was detected three times in order that the average deviation was less than 10%.
3 Results and discussion
3.1 Results of chemical mutagenesis
3.1.1 Determination of death rate of chemical mutagenesis
The results of mortality rate of mutant strains are shown in Table 1. Compared with the original strains, the mortality rate of mutant strains of Penicillium simplicissimum was between 60% and 100%. In this experiment, the mutant colonies of the death rate of 75.46% were chosen for obtaining more positive mutation, in other words, the chemical mutant strains had been chosen through 30 min reaction for the next screening culture, named C strains.
Table 1 Lethal rate of chemical mutation

3.1.2 Results of screening
The chemical mutant strains of better growth which were chosen in this experiment were named as C1, C2, C3, … and C13. The fade of PDA-RBBR screening plate are shown in Table 2. By comparing with the PDA-RBBR flat fading, the situation of the fade of the plate by C1, C5 and C9 strains is better than that of the initial strains, so the initial strains, C1, C5 and C9 strains were selected for solid fermentation.
3.1.3 Laccase activity
Laccase activity of Penicillium simplicissimum was detected in the fermentation process. The time courses for extracellular laccase are shown in Fig. 1. The laccase activity of original microorganism increased continuously and reached the maximum of 3.75 U/g on day 6, and then decreased gradually until it remained stable after 12 d. The time courses for laccase activity of C5 strains were similar with original microorganism, and reached the maximum of 4.05 U/g on day 6, a litter ahead of original microorganism. The laccase activities of C1 strains and C9 strains in the first 8 d were instable and reached the maximum on day 8. And on day 14, there was another peak of laccase activity of C9 strains. But the maximum laccase activities of C1 and C9 strains were less than the maximum activity of the original strain. So. C5 strains were selected for UV mutation.
Table 2 Fading of PDA-RBBR screening plate
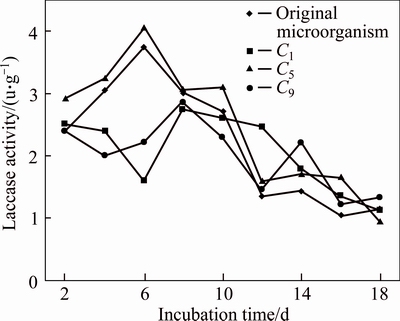
Fig. 1 Laccase activities of original microorganism and chemical mutated strains during fermentation
3.2 Results of UV mutagenesis
3.2.1 Determination of death rate of UV mutagenesis
C5 strains were selected for UV mutation. The results of mortality rate of mutant strains are shown in Table 3. Compared with C5 strains, the mortality rate of mutant strains of Penicillium simplicissimum was between 70% and 90%. In this experiment, for the next screening culture, the mutant colonies of the death rate of 80% were chosen, in other words, we had chosen the mutant strains which were irradiated for 4 min.
3.2.2 Results of screening
The UV mutant strains of better growth which had been chosen in this experiment were named as C5E1, C5E2, C5E3 … and C5E10. The fading of PDA-RBBR screening plate is shown in Table 4. By comparing with the PDA-RBBR flat fading, we selected C5, C5E4, C5E7 and C5E9 strains for the next solid fermentation.
Table 3 Lethal rate of UV mutation

Table 4 Fading of PDA-RBBR screening plate
3.2.3 Laccase activity
The trends of laccase activity in the fermentation process of Penicillium simplicissimum over time are shown in Fig. 2. It was obvious that the trend of C5E4 strains was similar with C5 strains and laccase activities of C5E4 strains were higher than those of C5 strains in the most of the time. On day 6, the maximum laccase activity of C5E4 reached 4.80 U/g which was higher than the maximum laccase activity of C5 strains. The laccase activities of C5E7 and C5E9 strains were lower than the activity of C5 in the early stages of cultivation. After being cultured for 10 d, both of them increased significantly, and on the late stages of cultivation enzyme activities of C5E7 and C5E9 strains were higher than that of C5 strains. But their maximum activities were lower than the maximum activity of C5. So, C5E4 strains were selected.
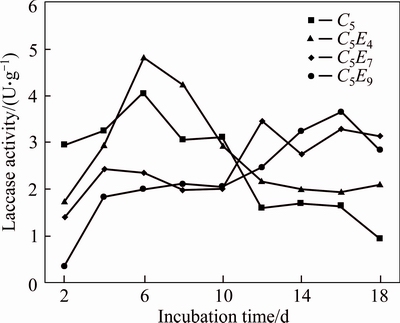
Fig. 2 Laccase activities of C5 and UV mutated strains during fermentation
3.3 Genetic stability of mutant strains
In order to study the genetic stability of laccase production of mutant strains, Five generations of original microorganism, C5 and C5E4 strains were cultured, and the laccase activities of the strains were measured. The maximum of laccase production of subcultures of strains is shown in Fig. 3. C5E4 strains can product laccase of the five subcultures stably and the maximum of laccase of C5E4 strain was over 18% higher than the maximum of laccase of original microorganism.
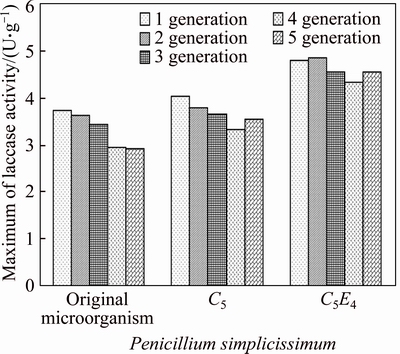
Fig. 3 Maximum of laccase production of strains original microorganism, C5 and C5E4
3.4 Discussion
The strains of the death rate of 70%-80% in chemical mutagenesis and UV mutagenesis process were chosen, because there is more positive mutant strains generated when lethal mutation rate is 70%-80%, according to breeders’ long-term research and practical experience. So the strains which the death rate were 75.46% and 80% in chemical mutagenesis and UV mutagenesis respectively were selected in this experiment,.
Because the laccase from Penicillium simplicissimum can degrade anthraquinone dye RBBR [26], the strains were selected according to the fading of PDA-RBBR in the screening process. In the process of chemical mutagenesis, the conditions of the fade of PDA-RBBR screening plate by the selected strains C1, C5, C9 were better than the original strains. And in the course of UV-induced mutation, compared with the C5 strain, the conditions of the fade of PDA-RBBR by the selected strains C5E4, C5E7 and C5E9 were better. But both laccase and lignin peroxidase from Penicillium simplicissimum can fade RBBR [26, 27], so the selected strains should be cultured in solid fermentation substrate and the laccase of the strains should be detected with ABTS to select a strain of higher laccase activity.
The laccase activities of C1, C5, C9, C5E4 and the original strains were increased and then decreased to a stable level in the experiment. This is because the biomass and the laccase of Penicillium simplicissimum were less in the initial phase of incubating. The fungal biomass and laccase activity were increased over the incubating days. Maybe due to the metabolites produced, enzyme production of Penicillium simplicissimum was inhibited, the laccase activity showed downward trend, with the increase of cultivation time, although the biomass of Penicillium simplicissimum was still on the rise [28]. So the growth process and enzyme production process of Penicillium simplicissimum did not correspond. The laccase activity of C5E7 and C5E9 were different with the original strains, they showed instability, increased first and then decreased, up again and declined at the end, and the laccase activities from late cultured Penicillium simplicissimum were higher than those from the previous cultured. The reasons causing this phenomenon may be uncertainty of mutation.
4 Conclusions
Chemical mutation and UV mutation were combed in Penicillium simplicissimum to obtain mutant strains of high-yield laccase. By PDA-RBBR flat fading screening and solid state fermentation screening, a high-yield laccase strain of Penicillium simplicissimum C5E4 was obtained. The maximum of laccase of C5E4 which was determined by ABTS was 4.80 U/g and 18% higher than original strains. By transfer of culture, the genetic stability of strains had been verified. Preliminary experiments results showed that the techniques of chemical mutation and UV mutation can be used as a means of improving the laccase activity of Penicillium simplicissimum.
References
[1] ALEXANDRE G, ZHULIN I B. Laccases are widespread in bacteria [J]. Trends in Biotechnology, 2000, 18(2): 41-42.
[2] LAI Cui, ZENG Guang-ming, HUANG Dan-lian, ZHAO Mei-hua, WEI Zhen, HUANG Chao, XU Piao, LI Ning-jie, ZHANG Chen, CHEN Ming, LI Xue, LAI Ming-yong, HE Yi-bin. Synthesis of gold- cellobiose nanocomposites for colorimetric measurement of cellobiase activity [J]. Spectrochimica Acta Part A, 2014, 132(21): 369-374.
[3] LITTHAUER D. Purification and kinetics of a thermostable laccase from Pycnoporus sanguineus (SCC 108) [J]. Enzyme & Microbial Technology, 2007, 40(4): 563-568.
[4] XU Li-jing, WANG He-xiang, NG T B. A Laccase with HIV-1 reverse transcriptase inhibitory activity from the broth of mycelial culture of the mushroom lentinus tigrinus [J]. Journal of Biomedicine & Biotechnology, 2012(1): 536725-536731.
[5] MUNK L, SITARZ A K, KALYANI D C, MIKKELSEN J D, MEYER A S. Can laccases catalyze bond cleavage in lignin [J]. Biotechnology Advances, 2015, 33(1): 13-24.
[6] ZHOU Yun, DENG Tian-fu, PAN Chen-yuan, CHEN Chun-run, MO Jian-chu. Purification of a laccase from fungus combs in the nest of Odontotermes formosanus [J]. Process Biochemistry, 2010, 45(7): 1052-1056.
[7] BIRHANLI E, YESILADA O. Increased production of laccase by pellets of Funalia trogii ATCC 200800 and Trametes versicolor ATCC 200801 in repeated-batch mode [J]. Enzyme and Microbial Technology, 2006, 39(6): 1286-1293.
[8] FAN Fang-fang, ZHUO Rui, SU Sun, WAN Xia, JIANG Mu-lan, ZHANG Xiao-yu, YANG Yang. Cloning and functional analysis of a new laccase gene from Trametes sp. 48424 which had the high yield of laccase and strong ability for decolorizing different dyes [J]. Bioresource Technology, 2011, 102(3): 3126-3137.
[9] MURUGESAN K, NAM I H, KIM Y M, CHANG Y S. Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture [J]. Enzyme & Microbial Technology, 2007, 40(7): 1662-1672.
[10] DONNA D T, TIWAR R, SAH A K, RAGHUKUMAR C. Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes [J]. Enzyme and Microbial Technology, 2006, 38(3, 4): 504-511.
[11] QIU Wei-hua, CHEN Hong-zhang. An alkali-stable enzyme with laccase activity from entophytic fungus and the enzymatic modification of alkali lignin [J]. Bioresource Technology, 2008, 99(13): 5480-5484.
[12] SREBOTNIK E, BOISSON J N. Peroxidation of linoleic acid during the oxidation of phenols by fungal laccase [J]. Enzyme & Microbial Technology, 2005, 36(5, 6): 785-789.
[13] JING De-bin, WANG Jing-hua. Controlling the simultaneous production of laccase and lignin peroxidase from Streptomyces cinnamomensis by medium formulation [J]. Biotechnology for Biofuels, 2012, 5(1): 15-21.
[14] ZENG Guang-ming, YU Hong-Yan, HUANG Hong-Li, HUANG Dan-lian, CHEN Yao-ning, HUANG Guo-he. Laccase activities of a soil fungus Penicillium simplicissimum in relation to lignin degradation [J]. World Journal of Microbiology & Biotechnology, 2006, 22(4): 317-324.
[15] YU Hong-yan, ZENG Guang-ming, HUANG Guo-he, HUANG Dan-lian, CHEN Yao-ning. Lignin degradation by Penicillium simplicissimum [J]. Environmental Science, 2005, 26(2): 167-171. (in Chinese)
[16] SHEN Ying, HU Tian-jue, ZENG Guang-ming, WU Juan-juan, HUANG Chao, LIU Hui. Improving lignin degradation ability of Penicillium simplicissimum by UV induced protoplast mutagenesis [J]. China Environmental Science, 2012, 32(3): 485-491. (in Chinese)
[17] LIU Jian-xiao, ZHOU Wen-Jing, GONG Ji-lai, TANG Lin, ZHANG Yi, YU Hong-yan, WANG Bin, XU Xiang-min, ZENG Guang-ming. An electrochemical sensor for detection of laccase activities from Penicillium simplicissimum in compost based on carbon nanotubes modified glassy carbon electrode [J]. Bioresource Technology, 2008, 99(18): 8748-8751.
[18] XU Ran, TANG Rong-zhi, ZHOU Qi-jun, LI Feng-ting, ZHANG Bing-ru. Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes [J]. Chemical Engineering Journal, 2015, 262(262): 88-95.
[19] XU Xue-qin, LI Xiao-ming, YANG Qi, LIAO De-xiang, ZENG Guang-ming, ZHANG Yu, LIU Jing-jin. Biosorption of lead and copper ions by Penicillium simplicissimum immobilized on a loofa sponge immobilized biomass [J]. Acta Scientiae Circumstantiae, 2008, 28(1): 95-100. (in Chinese)
[20] RAVELET C, KRIVOBOK S, SAGE L, STEIMAN R. Biodegradation of pyrene by sediment fungi [J]. Chemosphere, 2000, 40(5): 557-563.
[21] TOYAMA T, FURUKAWA T, MAEDA N, INOUE D, SEI K, MORI K, KIKUCHI S, IKE M. Accelerated biodegradation of pyrene and benzo[a]pyrene in the Phragmites australis rhizosphere by bacteria-root exudate interactions [J]. Water Research, 2011, 45(4): 1629-1638.
[22] CHAINEAU C H, MOREL J, DUPONT J, BURY E, OUDOT J. Comparison of the fuel oil biodegradation potential of hydrocarbon-assimilating microorganisms isolated from a temperate agricultural soil [J]. Science of the Total Environment, 1999, 227(2, 3): 237-247.
[23] LIU Hui, HU Tian-jue, ZENG Guang-ming, WU Juan-juang, YANG Chun-ping, ZHANG Ying, SHEN Ying. Power generation using two kinds of phenolic compounds as substrates in MFC [J]. Chinese Journal of Environmental Engineering, 2012, 6(1): 212-217. (in Chinese)
[24] HU Tian-jue, WU Juan-juan, ZENG Guang-ming, LIU Hui, ZHANG Ying, HUANG Dan-lian, YU Bing, SHEN Ying. Research on the degradation effect of contaminants like Phenols and Anilines by Using Penicillium simplicissimum [J]. Journal of Hunan University, 2011, 38(4): 61-65. (in Chinese)
[25] ZHANG Chen, LAI Cui, ZENG Guang-ming, HUANG Dan-lian, YANG Chun-ping, WANG Yang, ZHOU Yao-yu, CHENG Min. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution [J]. Water Research, 2016, 95: 103-112.
[26] HOU Hong-man, ZHOU Ji-ti, WANG Jing, DU Cui-hong, YAN Bin. Characteristics of laccase from white-rot fungus pleurotus ostreatus strain 3.42 and its decolorization effect on anthraquinone dye [J]. Chemistry & Industry of Forest Products, 2004, 24(1): 48-52. (in Chinese)
[27] MUNOZ C, GUILLEN F, MARTINEZ AT, MARTINEZ M J. Laccase isoenzymes of Pleurotus eryngii: Characterization, catalytic properties, and participation in activation of molecular oxygen and Mn(II) oxidation [J]. Applied & Environmental Microbiology, 1997, 63(6): 2166-2174.
[28] ZHANG Chen, LAI Cui, ZENG Guang-ming, HUANG Dan-lian, TANG Lin, YANG Chun-ping, ZHOU Yao-yu, QIN Lei, CHENG Ming. Nanoporous Au-based chronocoulometric aptasensor for amplified detection of Pb2+ using DNAzyme modified with Au nanoparticles [J]. Biosensors and Bioelectronics, 2016, 81: 61-67.
(Edited by HE Yun-bin)
Cite this article as:
LI Xue, LI Fei, LAI Cui, HUANG Jin-hui, PANG Ya, LUO Kun, LIAO Xing-sheng. Activities of laccase produced by a strains Penicillium simplicissimum induced by chemical agentia and UV radiation [J]. Journal of Central South University, 2017, 24(9): 1953–1958.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-017-3603-9Foundation item: Projects(51178172, 51308076, 51408206, 51578222) supported by the National Natural Science Foundation of China; Project(113049A) supported by the Ministry of Education of China
Received date: 2016-02-29; Accepted date: 2016-07-11
Corresponding author: LI Xue, PhD; Tel: +86-731-84261421; E-mail: gina0131@163.com; LI Fei, PhD; Tel: +86–27–88385169; E-mail: Lifei@zuel.edu.cn
Abstract: Penicillium simplicissimum was cultured and preserved on the potato dextrose agar (PDA) medium. PDA-RBBR (Remazal Brilliant Blue R) medium was used for the screening of the strains, which is able to produce enzymes. After the mutation process in Penicillium simplicissimum induced by chemical reagent and ultraviolet radiation, a high laccase-producing strains Penicillium simplicissimum was obtained. When 5 mL diethyl sulfate (2%) was mixed along with 5 mL spore suspension for 30 min, chemical mutagenesis reached its best condition. And the optimum conditions of UV mutagenesis were that spore suspension was irradiated for 4 min under 15 W UV lamp at a distance of 30 cm. The highest activity of C5E4 strains was 4.80 U/g over 18% higher than the maximum laccase activity of original microorganism. Five generations of the mutant strains were cultured, and the laccase activity of the strains was measured. The result showed that C5E4 strains can product laccase of the five subcultures stably.


