
Biosorption of cadmium(Ⅱ) from aqueous solutions by industrial fungus Rhizopus cohnii
LUO Jin-ming(罗金明)1, XIAO Xiao(肖 潇)2, 3, LUO sheng-lian(罗胜联)4
1. Faculty of Life Science and Technology, Central South University of Forestry and Technology,
Changsha 410004, China;
2. College of Environmental Science and Engineering, Hunan University, Changsha 410082, China;
3. Key Laboratory of Environmental Biology and Pollution Control of Ministry of Education, Hunan University,
Changsha 410082, China;
4. State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan University, Changsha 410082, China
Received 23 November 2009; accepted 8 April 2010
Abstract:
An important filamentous industrial fungus, Rhizopus cohnii (R. cohnii), was used as an efficient biosorbent for removing cadmium from wastewater. The sorption conditions, such as pH, the dose of biomass and the initial concentration of cadmium were examined. Two kinds of adsorption models were applied to simulate the biosorption data. The uptake of cadmium was higher in weak acid condition than in strong acid condition. Nearly no sorption of cadmium occurred when the pH value was lower than 2.0. Biosorption isothermal data could be well simulated by both Langmuir and Freundlich models. Langmuir simulation of the biosorption showed that the maximum uptake of cadmium was 40.5 mg/g (0.36 mmol/g) in the optimal conditions, which was higher than many other adsorbents, including biosorbents and activated carbon. In addition, the reusability results showed that after five times of sorption and desorption process, the sorption capacity of R. cohnii could still maintain nearly 80%, confirming its practical application in cadmium treatment. Fourier transform infrared spectrum revealed that carboxyl, amino and hydroxyl groups on biosorbent R. cohnii surface were responsible for the biosorption of cadmium.
Key words:
biosorption; Rhizopus cohnii; biosorbent; cadmium;
1 Introduction
Cadmium is one of the most toxic metals found in effluents discharged from industries involved in metal plating, metallurgical alloying, mining, ceramics and other industrial activities. It is non-biodegradable and tends to accumulate in living organisms, causing significant threats to both the environment and public health[1]. Many laws and rules are set up to restrict this pollutant releasing into environment to pollute the air, soil and water in China.
Many physicochemical strategies, such as filtration, chemical precipitation, electrochemical treatment, ion exchange, oxidation or reduction, reverse osmosis, and evaporation recovery, have been developed to remove heavy metals, including cadmium, from polluted water [1-4]. However, there are some disadvantages for traditional physicochemical methods to treat cadmium- polluted wastewater, such as expensive cost, low efficiency, labor-intensive operation, and lack of selectivity in the treating process[5].
Compared with the conventional methods, biosorption is considered to be a promising option to solve the environmental pollution of heavy metals. The major advantages of biosorption include low cost, high efficiency of heavy metal removal from diluted solutions, regeneration of the biosorbent and the possibility of metal recovery[6]. The promising challenge to traditional operation attracts people to exploit more bioresources as biosorbent in this research field.
Fungi are widely used in a variety of industrial fermentation processes which could serve as an economical and constant supply source of biomass to remove metal ions from wastewater. Fungi can also easily grow in substantial amounts using unsophisticated fermentation techniques and inexpensive growth media. Therefore, a fungal biomass could serve as an economical method for removal or recovery of metal ions from aqueous solutions[7]. Some literatures report that many kinds of fungi are capable of removing heavy metals during sewage treatment, such as fungus Aspergillus niger[7], fungus Phanerochaete chrysosporium[8], white-rot fungus[9], fungal biomass of Mucor racemosus[10] and the by products of brown-rot fungus L. edodes[11].
Rhizopus species are important filamentous fungi that have been widely used in modern industries for processing traditional fermented foods, industrial enzyme production and organic acids such as fumaric and lactic acid production[12-13]. Abundance of this kind of biomaterial was thrown away after application. This biomaterial contains plenty of chitin and lignin, which are very efficient in heavy metals biosorption[14]. Plenty of myceliums in the byproduct have many important bene?ts for human beings, such as degrading organopollutants[15] and sorbing heavy metals such as chromium[16].
This study aimed at using the industrial fungus Rhizopus cohnii (R. cohnii) to remove cadmium in simulated wastewater. The factors that affect biosorption capacity, such as pH, the dosage and reusability of biosorbent and the initial cadmium concentration were examined. Freundlich and Langmuir isotherm models were used to simulate the biosorption characterization. Moreover, Fourier transform infrared spectrum was employed to understand the biosorption mechanism.
2 Experimental
2.1 Preparation of reagents and medium
All reagents used were of analytical grade and purchased from Shanghai Pharmaceutical Co. Ltd. in China. The 1 000 mg/L Cd(Ⅱ) stock solution was prepared by dissolving the exact quantities of the 3CdSO4?8H2O in deionized-distilled water. The working concentration of Cd(Ⅱ) solution was prepared from suitable serial dilution of the stock solution. The deionized-distilled water used in this experiment was obtained from a Milli-Q system (Millipore, USA).
The yeast peptone sucrose (YPS) medium contained 3.0 g/L yeast extract, 10 g/L peptone and 20 g/L sucrose. The pH of the medium was adjusted to 4.5.
2.2 Preparation of adsorbents
R. cohnii, an industrial fungus presented by Hunan Light Industry Research Institute, China, was cultivated in the YPS medium at 303 K. After incubation for 3 days, the mycelia were washed several times with deionized- distilled water. The biomass were killed by autoclaving (15 lb, 394 K) for 20 min, and then dried at 343 K until mass was kept constant. Subsequently, the dried mycelia were crushed into fractions. The powdered biomass residues obtained (particle size between 0.45 and 1.0 mm) were referred to as “biosorbent R. cohnii” in this work. The activated carbon with similar diameter was obtained from Shanghai Xingchang Activated Carbon Co., Ltd, China. The adsorbents were all stored in desiccators for the following experiments.
2.3 Analytical technique
The concentrations of cadmium ions were determined by the flame atomic absorption spectrometry (FAAS) using Z2000 polarized zeeman atomic absorption spectrophotometer (Hitachi, Japan). The hollow cathode lamp was operated at 5 mA and the analytical wavelength was set at 228.8 nm.
2.4 Batch biosorption and desorption experiments
20, 50 and 100 mg/L of cadmium solution were conducted to determine optimal pH, contact time and dosage (dry mass). Biosorbent R. cohnii was mixed with Cd(Ⅱ) solution and agitated in an incubator at 150 r/min, 298 K. The effect of pH was investigated in the range of 1.5-6.5 at the dosage of 1.0 g/L and contact time of 12 h (to ensure that equilibrium was reached). The pH values in the solutions were monitored by a FE20 pH electrode (Mettler Toledo, Shanghai, China). The kinetics of Cd(Ⅱ) sorption on biosorbent R. cohnii was also studied. The dosage was 1.0 g/L and the pH was adjusted to 4.5. Samples were taken and analyzed at the following time intervals: 0.25, 0.5, 1.0, 2.0, 4.0 and 6.0 h. The optimum dosage was examined in the range of 1.0-15.0 g/L.
The desorption study was conducted at the initial Cd(Ⅱ) concentration of 20, 50 and 100 mg/L under previously determined optimal adsorption conditions. After the biosorption of Cd(Ⅱ), the biosorbent was eluted with 20 mL 0.1 mol/L HNO3 for 1 h at 150 r/min. Then, the biosorbent was washed with deionized water till the pH of the eluate was in the range of 5.0-5.5. This cycle was repeated 5 times. Samples were taken after every adsorption and desorption process by filtering through 0.45 ?m filter units (Millipore, Ireland). The cadmium concentrations in the filtrate were analyzed with the methods mentioned above. All experiments were done three times, yielding an experimental error of less than 5%.
2.5 Effect of initial concentration and sorption capacities comparison
To estimate and compare the sorption capacities between biosorbent R. cohnii and activated carbon, the experiments were conducted at initial Cd(Ⅱ) concentrations from 10 to 1 000 mg/L, at previously determined optimum conditions (1 g/L dosage, pH 4.5 for biosorbent R. cohnii, pH 6.5 for activated carbon, 150 r/min, 298 K) and contacted for 2 h, respectively. Subsequently, the mixtures were filtered through 0.45 ?m filter units (Millipore, Ireland). The cadmium concentrations in the filtrate were analyzed with the methods mentioned above. The experimental data were processed via Langmuir and Freundlich isotherms. All experiments were done three times, yielding an experimental error less than 5%.
2.6 Fourier transform infrared analysis (FTIR)
To investigate the changes of functional groups during biosorption of cadmium by biosorbent R. cohnii, Fourier transform infrared analysis was employed to obtain the information associated with the biosorption mechanisms during the process.
Infrared spectra of the biosorbent before and after adsorption were acquired by a FTIR (Nicolet 5700 Thermo, USA). The mass ratio of sample to KBr used for the preparation of the disks was 1:100.
3 Results and discussion
3.1 Effect of pH
The pH level is one of the most important parameters on biosorption of metal ions from aqueous solutions[7,17-18]. Regarding R. cohnii, its high content of ionizable groups (e.g. carboxyl groups from mannuronic and guluronic) on the cell wall polysaccharides, makes it, at least in theory, very liable to influence the pH of medium[6]. As shown in Fig.1, barely any biosorption was observed for a pH less than 2.0. The cadmium uptake of biosorbent R. cohnii increased with the pH increasing from 2.0 to 4.5 and then reached a plateau in the pH range of 4.5-6.5. Similar trends were also observed by DA COSTA and DEFRANCA[19] and CRUZ et al[6].
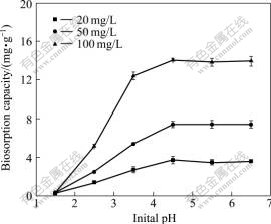
Fig.1 Effect of pH on Cd(Ⅱ) biosorption capacity using biosorbent R. cohnii at different Cd(Ⅱ) concentrations (contact time 12 h, dosage of 1 g/L at 298 K)
The pH values investigated were less than 7.0 since insoluble cadmium hydroxide started precipitating from the solution at higher pH values, making true sorption studies impossible. The biosorption capacities of biosorbents depend on the available binding sites provided by the functional groups existing on the surface of the biosorbents[1]. Such sites were not available due to competition between Cd2+ and H3O+ ions when pH< 2.0. With the acidity decreasing in the solution, the deprotonation of acid functional groups, such as carboxyl, phosphonate and phosphodiester, was strengthened and the attraction increased between negative charge on biomass and positive metal cations[1, 11].
3.2 Kinetic studies
As can been seen in Fig.2, there was an increase in the adsorption of cadmium with the increase of sorption time. The equilibrium was achieved after 2 h and no remarkable changes were observed for longer reaction time. The biosorption capacities of biosorbent R. cohnii were 3.8, 7.1 and 13.9 mg/g for the initial cadmium concentration of 20, 50 and 100 mg/L, respectively.
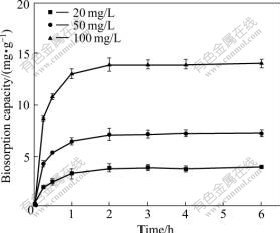
Fig.2 Effect of sorption time on Cd(Ⅱ) biosorption capacity using biosorbent R. cohnii at different initial concentrations at 298 K and pH 4.5
The biosorption kinetics of heavy metal ions consisted of two phases: an initial rapid phase where the biosorption was rapid and contributed significantly to the equilibrium biosorption, and a slower second phase whose contribution to the total metal biosorption was relatively small. The first phase of biosorption kinetics lasted for almost 1 h. The trend of Cd(Ⅱ) biosorption was typical of metal binding to biomass by means of physicochemical interactions. Such particular behavior could be due to the non-homogeneity of the biomass surface which possesses functional groups differing in dissociability and in cadmium adsorption rates[20].
There have been several reports[7, 17-18, 21] on the use of different kinetic models to adjust the experimental data of heavy metals adsorption on biosorbents. One of them is the pseudo-?rst-order kinetic model that considers the occupation rate of adsorption sites is proportional to the number of unoccupied sites. Its equation[6] can be expressed as
ln(Qe-Qt)=-K1t+lnQe (1)
where Qt and Qe are the amounts of metal ions adsorbed by the biosorbent at a given time of t and at equilibrium, respectively; K1 is the biosorption rate constant. Linear plots of ln(Qe-Qt) vs t indicate the applicability of this kinetic model.
The other model is based on the fact that the cadmium ions displace alkaline-earth ions (Ca2+ or Mg2+) from the biosorption sites of biosorbents and, therefore, with respect to the biosorption sites, the metal ions sorption can be considered to be a pseudo-second-order reaction[1]. The kinetics can be modeled assuming that the occupation rate of adsorption sites is proportional to the square of the number of unoccupied sites. Its equation[6] can be expressed as
![]() (2)
(2)
where K2 is the constant rate of second-order biosorption. The plot t/Qt vs t should give a straight line if second- order kinetics are applicable.
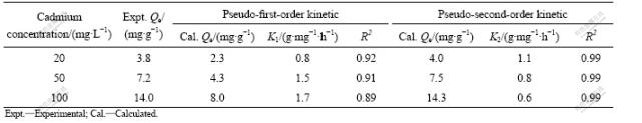
To evaluate the biosorption kinetics of cadmium ions, the pseudo-first-order and pseudo-second-order kinetic models were used to fit the experimental data. As shown in Table 1, the relative coefficient (R2) values of pseudo-second-order kinetics were all 0.99 which were better than those of pseudo-first-order kinetics (0.92 for 20 mg/L, 0.91 for 50 mg/L and 0.89 for 100 mg/L). Moreover, the Qe values predicted from pseudo-second- order kinetics were 4.0, 7.5 and 14.3 mg/g with initial concentrations of 20, 50 and 100 mg/L, respectively. These were closer to the experimental Qe values (3.8 mg/g for 20 mg/L, 7.2 mg/g for 50 mg/L and 14.0 mg/g for 100 mg/L) than the results from the pseudo-first- order kinetics, suggesting the cadmium biosorption mechanism was ion exchange. The cadmium ions displace alkaline-earth ions (Ca2+ or Mg2+) from the biosorption sites of biomass[1, 6].
Table 1 Biosorption rate constants and Qe values from pseudo-?rst-order and pseudo-second-order kinetics for biosorption of cadmium on biosorbent
3.3 Effect of dosage and reusability
The economic possibility of a biosorbent is the major issue in its application. This could be estimated by the dosage and reusability of the biosorbent.
The dosage effect of biosorbent R. cohnii was investigated at initial cadmium concentrations of 20, 50 and 100 mg/L. The uptake of Cd(Ⅱ) decreased with increasing dosages. The maximum uptakes of cadmium were 3.6, 7.3 and 13.7 mg/g at the dosages of 1 g/L (Fig.3).
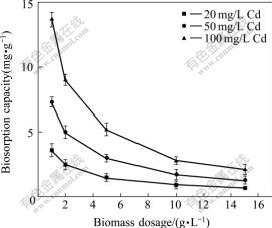
Fig.3 Effect of dosage on Cd(Ⅱ) biosorption capacity by using biosorbent R. cohnii at different initial concentrations at 298 K and pH 4.5
The lower the biomass dosage was, the more feasible it would be to use. The biosorption capacity decreased with increasing the biomass dosage. Similar results were observed when other biomasses were employed as biosorbents to remove heavy metals, such as cadmium removal by a byproduct of L. edodes[11], lead, cadmium and zinc biosorption by Citrobacter strain MCMB-181[22] and biosorption of cadmium by black gram husk (Cicer arientinum)[23]. The number of binding sites available for adsorption was determined by the dose of biomass added to the solution. A higher biosorption capacity at a lower biomass dosage could be attributed to an increased ratio of metal to biosorbent, which decreased with an increase in biomass dosage[22].
The desorption experiment was performed by 0.1 mol/L HNO3 after adsorption. The results showed that he sorption capacity of biosorbent R. cohnii did not significantly decrease after five cycles. As shown in Table 2, over 80% of the sorption capacities remained after five adsorption-desorption cycles. The sorption capacity only decreased by 0.7, 0.9 and 2.3 mg/g at the initial Cd(Ⅱ) concentrations of 20, 50 and 100 mg/L, respectively. Moreover, the desorption efficiencies were all above 95% during five cycles at different initial Cd(Ⅱ) concentrations.
Table 2 Desorption of Cd(Ⅱ) from biosorbent R. cohnii at different initial concentrations of Cd(Ⅱ) for five times
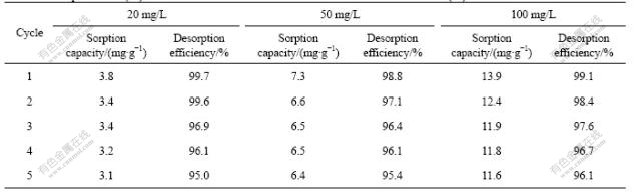
The application possibility of adsorbents depends not only on the sorptive capacity, but also on how well the biomass can be reused. The reusability of biosorbent R. cohnii was evaluated by repeating the adsorption and desorption experiments for five times. The high desorption efficiency indicated that nitric acid was the efficient desorbent agent for cadmium desorption (Table 2). Its efficiency is based on the competition between the protons and the cadmium ions adsorbed by the biosorbent, which will be released if the eluant concentration is high enough and there is not a steric impediment[24]. However, excessive amounts of hydrogen ions could reduce the biosorption capacity of the biomass[7]. Therefore, the reuse of the biomass in biosorption after elution of biosorbed cadmium ions will require hydrogen ions to be removed from the biomass. In this case, the biomass was regenerated by being washed with deionized water until the pH of the wash solution was in the range of 5.0-5.5. It was noticed that the first acid treatment was responsible for more than half of the biosorption decrease (Table 2). However, the decrease of sorption capacity at different initial Cd(Ⅱ) concentrations was not significant after five cycles. Similar deleterious effect of the acid treatment on the biomass was also observed when endophytic fungus (EF) Microsphaeropsis sp. LSE10 was used as biosorbent[1].
3.4 Effect of initial concentration
Analysis of equilibrium data is important for developing an equation that can be used to compare different biomaterials under different operational conditions and to design and optimize an operating procedure[6]. Several isotherm equations have been used for equilibrium models of biosorption systems. The two most commonly used isotherm equations, the Langmuir and Freundlich, have been applied in this study:
![]() (3)
(3)
where Qmax (mg/g) is the maximum amount of metal ion per unit mass of adsorbent to form a complete monolayer on the surface; b is the equilibrium adsorption constant, and is related to the affinity of the binding sites. Qmax represents a practical limiting adsorption capacity when the surface is fully covered with metal ions. It allows the comparison of adsorption performance, particularly in the case of where the adsorbent was not fully saturated.
![]() (4)
(4)
where K and n are the Freundlich constants’ system characteristics, indicating the adsorption capacity and adsorption intensity, respectively[1].
Both of them represent the equilibrium amount of metal removed (q) as a function of the equilibrium concentration (ρe) of metal ions in the solution, corresponding to the equilibrium distribution of ions between aqueous and solid phases as the initial concentration increases. To measure each isotherm, initial cadmium concentrations were varied while the biomass mass in each sample was kept constant. Equilibrium periods of 2 h for sorption experiments were used to ensure equilibrium conditions. This time was chosen considering the results of kinetics, which was determined previously.
To estimate the cadmium uptake capacity of biosorbent R. cohnii, we did not only compare its performance with other reported biosorbents, but also employed activated carbon, a widely and practically used adsorbent, as control adsorbents to run the sorption capacities comparison experiments.
The initial concentration of metal ions in the solution plays a key role as a driving force to overcome the mass transfer resistance between the aqueous and solid phases[25]. Therefore, the sorption capacity was expected to be higher with a higher initial concentration. As shown in Fig.4, the sorption capacity of both adsorbents increased with the equilibrium concentration of metal ions in the solution. The activated carbon performed better sorption capacity than biosorbent R. cohnii when the equilibrium cadmium concentration was below 100 mg/L. However, when the equilibrium cadmium concentration was higher than 100 mg/L, the situation was converse. This indicated the great potential application of R. cohnii as a biosorbent to treat cadmium wastewater at high concentrations.

Fig.4 Isotherm curves of Cd(Ⅱ) sorption by biosorbent R. cohnii and activated carbon at optimal conditions
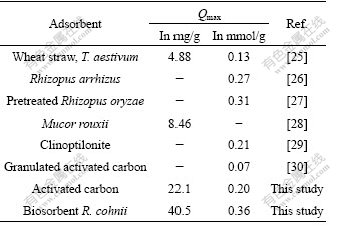
The maximum biosorption capacities of these two adsorbents for cadmium(Ⅱ) ions (Qmax) could be calculated from the Langmuir model. As can be seen in Table 3, the relative coefficient (R2) revealed that the sorption data of activated carbon and biosorbent R. cohnii fit both models, especially the Langmuir model (R2>0.99). The Qmax of biosorbent R. cohnii was 40.5 mg/g which was almost two times that of activated carbon (22.1 mg/g). In addition, it was higher than the Qmax values of other reported cadmium treatment adsorbents (Table 4).
Table 3 Langmuir and Freundlich isotherm parameters for Cd(Ⅱ) biosorption on biosorbent R. cohnii and activated carbon at optimal conditions
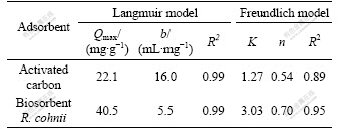
Table 4 Comparison of Langmuir estimated Qmax among different reported adsorbents for Cd(Ⅱ) biosorption
Comparison of the performances of different adsorbents is always achieved by comparing the parameters (such as Qmax and/or b) calculated from the same models (Langmuir and/or Freundlich models are most used). However, these comparisons only make sense under such preconditions: 1) The biosorbents should adsorb only the same adsorbate; 2) As the optimum performance conditions (external factors like pH, e.g.) for one adsorbent may be different for another, the optimal conditions should be determined previously and the sorption experiments should be done under their optimal conditions, respectively; 3) The data acquired from the experiments should be fitted to the calculated models (R2>0.9). Only the three pre- conditions mentioned above are satisfied, these data (parameters) are comparable. Consequently, the sorption capacities comparison experiments were performed at their optimum conditions which were determined previously (1 g/L adsorbents concentration, pH 6.5 for activated carbon, pH 4.5 for biosorbent R. cohnii, 150 r/min, 298 K). The data acquired from both the experiments and literatures were all fitted to Langmuir models (R2>0.99, Table 3).
3.5 Fourier transform infrared (FTIR) analysis
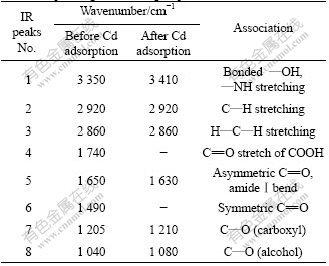
Numerous chemical groups have been proposed to be responsible for the biosorption of metals. They include carboxyl, sulphonate, hydroxyl and amino[31]. Their relative importance in metal sorption may depend on factors such as the quantity of sites, accessibility, chemical state and affinity between site and metal [32-33]. FTIR was an important tool to identify the functional groups. The vibrancy signals before and after biosorption of cadmium were different. The functional groups and the corresponding wave numbers were identified in this study compared with other research [33-35]. The assignment of FTIR bands and detailed wavenumber shifts for the biosorbent R. cohnii are summarized in Table 5. The shift of adsorption peak from 3 350 to 3 410 cm-1 indicated that the hydroxyl group had been changed from multimer to monopolymer or even dissociative state[36], which meant that the degree of the hydroxyl polymerization in biosorbent surface was decreased by the addition of Cd(Ⅱ). It offered more opportunity for Cd(Ⅱ) to be bound to the hydroxyl groups. Another change in the spectrum was the carboxyl. The adsorption peaks around 1 740 and 1 490 cm-1 were not observed after adsorption. In addition, the adsorption peaks were shifted from 1 650, 1 205 and 1 040 cm-1 to 1 630, 1 210 and 1 080 cm-1. This indicated that the carboxylic groups of cell wall biopolymers were active in metal sequestering[37].
Table 5 Association between bands observed in FTIR spectra and corresponding functional groups
4 Conclusions
1) Abundance biomass of Rhizopus cohnii, an important filamentous industrial fungus, is always throwing away after application. However, this kind of biomass was used as an efficient biosorbent for removing cadmium from wastewater for the first time.
2) The results of batch biosorption indicated that with the increase of pH in solution, the cadmium uptake of biosorbent R. cohnii was accordingly increased to the more extent with the initial concentration of 100 mg/L than with 50 and 20 mg/L. The optimal pH for cadmium biosorption was 4.5. The uptake of cadmium reached equilibrium within 2 h.
3) The application possibility of biosorbent R. cohnii was evaluated by dosage effect and reusability of the biosorbent. The biosorption capacity decreased with the increasing biomass dosage, and the optimal dosage for cadmium uptake of biosorbent R. cohnii was 1 g/L. Furthermore, over 80% of the sorption capacities remained after five adsorption and desorpion cycles. The desorption efficiencies were all above 95% during five cycles at different initial Cd(Ⅱ) concentrations, indicating that nitric acid was the efficient desorbent agent for cadmium desorption. This suggested that the biosorbent R. cohnii had great potential for practical application.
4) The biosorption capacity increased with the increase of cadmium concentration. The best simulated model to biosorption data was Langmuir model with the relative coefficient (R2) of 0.99 at pH around 4.5. The maximum uptake of cadmium was 40.5 mg/g which was higher than that of many other reported adsorbents. Fourier transform infrared analysis indicated that carboxyl, amino and hydroxyl groups on biosorbent R. cohnii surface were responsible for the biosorption of cadmium.
References
[1] XIAO X, LUO S L, ZENG G M, WEI W Z, WAN Y, CHEN L, GUO H J, CAO Z, YANG L X, CHEN J L, XI Q. Biosorption of cadmium by Endophytic Fungus (EF) Microsphaeropsis sp. LSE10 isolated from cadmium hyperaccumulator Solanum Nigrum L. [J]. Bioresource Technology, 2010, 101(6): 1668-1674.
[2] LUO Sheng-lian, YUAN Lin, CHAI Li-yuan, MIN Xiao-bo, WANG Yun-yan, FANG Yan, WANG Pu. Biosorption behaviors of Cu2+, Zn2+, Cd2+ and mixture by waste activated sludge [J]. Transaction of Nonferrous Metals Society of China, 2006, 16(6): 1431-1435.
[3] HE Yan, ZHOU Gong-ming. Research progress on excess sludge reduction technologies [J]. Environmental Technology, 2004(1): 39-42. (in Chinese)
[4] LIU Hui, CHAI Li-yuan, MIN Xiao-bo, WANG Yun-yan, YU Xia. Study and development of activated sludge treatment of heavy metal containing wastewater [J]. Industrial Water and Wastewater, 2004, 35(4): 9-11. (in Chinese)
[5] TARLEY C R T, ARRUDA M A Z. Biosorption of heavy metals using rice milling by-products: Characterisation and application for removal of metals from aqueous effluents [J]. Chemosphere, 2004, 54(7): 987-995.
[6] CRUZ C C V, DA COSTA, A C A, HENRIQUES C A, LUNA A S. Kinetic modeling and equilibrium studies during cadmium biosorption by dead Sargassum sp. biomass [J]. Bioresource Technology, 2004, 91(3): 249-257.
[7] KAPOOR A, VIRARAGHAVAN T, CULLIMORE D R. Removal of heavy metals using the fungus Aspergillus niger [J]. Bioresource Technology, 1999, 70(1): 95-104.
[8] SAY R, DENIZLI A, YAKUP ARICA M. Biosorption of cadmium (Ⅱ), lead (Ⅱ) and copper (Ⅱ) with the filamentous fungus Phanerochaete chrysosporium [J]. Bioresource Technology, 2001, 76(1): 67-70.
[9] ARICA M Y, KACAR Y, GENC O. Entrapmen of white-rot fungus Trametes versicolor in Ca-alginate beads: Preparation and biosorption kinetic analysis for cadmium removal from an aqueous solution [J]. Bioresource Technology, 2001, 80(2): 121-129.
[10] LIU T, LI H D, DENG L. The optimum conditions, thermodynamical isotherm and mechanism of hexavalent chromium removal by fungal biomass of Mucor racemosus [J]. World Journal of Microbiology and Biotechnology, 2007, 23(12): 1685-1693.
[11] CHEN G Q, ZENG G M, TANG L, DU C Y, JIANG X Y, HUANG G H, LIU H L, SHEN G L. Cadmium removal from simulated wastewater to biomass byproduct of Lentinus edodes [J]. Bioresource Technology, 2008, 99(15): 7034-7040.
[12] SOCCOL C R, MARTIN B, RAIMBAULT M, LEBEAULT J M. Breeding and growth of Rhizopus in raw cassava by solid state fermentation [J]. Applied Microbiology and Biotechnology, 1994, 41(3): 330-336.
[13] YU R, HANG Y D. Purification ad characterization of a glucoamylase from Rhizopus oryzae [J]. Food Chemistry, 1991, 40(3): 301-308.
[14] BAILEY S E, OLIN T J, BRICKA R M, ADRIAN D D. A review of potentially low-cost sorbents for heavy metals [J]. Water Research, 1999, 33(11): 2469-2479.
[15] MATHIALAGAN T, VIRARAGHAVAN T. Biosorption of pentachlorophenol from aqueous solutions by a fungal biomass [J]. Bioresource Technology, 2003, 100(2): 549-558.
[16] CHEN G. Q, ZENG G M, TU X, NIU C G., HUANG G H, JIANG W. Application of a by-product of Lentinus edodes to the bioremediation of chromate contaminated water [J]. Journal of Hazardous Materials, 2006, 135(1/3): 249-255.
[17] AKSU Z. Equilibrium and kinetic modeling of cadmium (Ⅱ) biosorption by C. vulgaris in a batch system: Effect of temperature [J]. Separation and Purification Technology, 2001, 21(3): 285-294.
[18] ZHANG, L, ZHAO L, YU Y, CHEN C. Removal of lead from aqueous solution by non-living Rhizopus nigricans [J]. Water Research, 1998, 32(5): 1437-1444.
[19] DA COSTA A C A, DEFRANCA F P. Cadmium sorption by biosorbent seaweeds: Adsorption isotherms and some process conditions [J]. Separation Science and Technology, 1996, 31(17): 2373-2393.
[20] MATHEICKAL J T, YU Q, WOODBURN G M. Biosorption of cadmium (Ⅱ) from aqueous solution by pre-treated biomass of marine algae Durvillaea potatorum [J]. Water Research, 1999, 33(2): 335-343.
[21] BENGUELLA B, BENAISSA H. Cadmium removal from aqueous solution by chitin: Kinetic and equilibrium studies [J]. Water Research, 2002, 36(10): 2463-2474.
[22] PURANIK P R, PAKNIKAR K M. Biosorption of lead, cadmium and zinc by citrobacter strain MCMB-181: Characterization studies [J]. Biotechnology Progress, 1999, 15(2): 228-237.
[23] SAEED A, IQBAL M. Bioremoval of cadmium from aqueous solution by black gram husk (Cicer arientinum) [J]. Water Research, 2003, 37(14): 3472-3480.
[24] HERRERO R, LODEIRO P, ROJO R, CIORBA A, RODR?GUEZ P, MANUEL E, SASTRE DE VICENTE. The effciency of the red alga Mastocarpus stellatus for remediation of cadmium pollution [J]. Bioresource Technology, 2008, 99(10): 4138-4146.
[25] DANG V B H, DOAN H D, DANG-VU T, LOHI A. Equilibrium and kinetics of biosorption of cadmium(Ⅱ) and copper(Ⅱ) ions by wheat straw [J]. Bioresource Technology, 2009, 100(1): 211-219.
[26] TOBIN J M, L’HOMME B, ROUX J C. Immobilisation protocols and effects on cadmium uptake by Rhizopus arrhizus biosorbents [J]. Biotechnology Techniques, 1993, 7(10): 739-744.
[27] YIN P, YU Q, JIN B, LING Z. Biosorption removal of cadmium from aqueous solution by using pretreated fungal biomass cultured from starch wastewater [J]. Water Research, 1999, 33(8): 1960-1963.
[28] YAN G, VIRARAGHAVAN T. Heavy metal removal from aqueous solution by fungus Mucor rouxii [J]. Water Research, 2003, 37(18): 4486-4496.
[29] CURKOVIC L, STEFANOVIC S C, FILIPAN T. Metal ion exchange by natural and modi?ed zeolites [J]. Water Research, 1997, 31(6): 1379-1382.
[30] RAMOS R L, MENDEZ J R R, BARRON J M, RUBIO L, CORONADO R G M. Adsorption of Cd(Ⅱ) from aqueous solution onto activated carbon [J]. Water Science and Technology, 1997, 35(7): 205-211.
[31] VOLESKY B. Biosorption and me [J]. Water Research, 2007, 41(18): 4017-4029.
[32] XIAO X, YANG X, LIU T, CHEN Z, CHEN L L, LI H D, DENG L. Preparing a highly specific inert immunomolecular-magnetic beads for rapid detection and separation of S. aureus and group G Streptococcus [J]. Applied Microbiology and Biotechnology, 2007, 75(5): 1209-1216.
[33] MURPHY V, HUGHES H, MCLOUGHLIN P. Cu(Ⅱ) binding by dried biomass of red, green and brown macroalgae [J]. Water Research, 2007, 41(4): 731-740.
[34] LI H D, ZHAO L, LIU T, XIAO X, PENG Z H, DENG L. A novel technology for biosorption and recovery hexavalent chromium in wastewater by bio-functional magnetic beads [J]. Bioresource Technology, 2008, 99(14): 6271-6279.
[35] GHINWA N, CHRISTIAN M, JACQUE B, BOHUMIL V. Lead biosorption study with Rhizopus arrhizus using a metal-based titration technique [J]. Journal of Colloid and Interface Science, 2005, 292(2): 537-543.
[36] KELLNER R, MERMET J M, OTTO M. Analytical chemistry [M]. New York: Wiley-VCH Verlag GmbH Press, 1998: 824.
[37] DAVIS T A, LLANES F, VOLESKY B, MUCCI A. Metal selectivity for Sargassum spp. and their alginates in relation to their α-L-guluronic acid content and conformation [J]. Environmental Science and Technology, 2003, 37(2): 261-267.
Foundation item: Project(50830301) supported by the National Natural Science Foundation of China; Project(50725825) supported by the National Science Fund for Distinguished Young Scholars of China
Corresponding author: LUO sheng-lian; Tel: +86-731-88821967; E-mail: sllou@hnu.cn
DOI: 10.1016/S1003-6326(09)60264-8
(Edited by LI Xiang-qun)
Abstract: An important filamentous industrial fungus, Rhizopus cohnii (R. cohnii), was used as an efficient biosorbent for removing cadmium from wastewater. The sorption conditions, such as pH, the dose of biomass and the initial concentration of cadmium were examined. Two kinds of adsorption models were applied to simulate the biosorption data. The uptake of cadmium was higher in weak acid condition than in strong acid condition. Nearly no sorption of cadmium occurred when the pH value was lower than 2.0. Biosorption isothermal data could be well simulated by both Langmuir and Freundlich models. Langmuir simulation of the biosorption showed that the maximum uptake of cadmium was 40.5 mg/g (0.36 mmol/g) in the optimal conditions, which was higher than many other adsorbents, including biosorbents and activated carbon. In addition, the reusability results showed that after five times of sorption and desorption process, the sorption capacity of R. cohnii could still maintain nearly 80%, confirming its practical application in cadmium treatment. Fourier transform infrared spectrum revealed that carboxyl, amino and hydroxyl groups on biosorbent R. cohnii surface were responsible for the biosorption of cadmium.
Water Research, 1999, 33(2): 335-343." target="blank">[20] MATHEICKAL J T, YU Q, WOODBURN G M. Biosorption of cadmium (Ⅱ) from aqueous solution by pre-treated biomass of marine algae Durvillaea potatorum [J]. Water Research, 1999, 33(2): 335-343.
[31] VOLESKY B. Biosorption and me [J]. Water Research, 2007, 41(18): 4017-4029.
Water Research, 2007, 41(4): 731-740." target="blank">[33] MURPHY V, HUGHES H, MCLOUGHLIN P. Cu(Ⅱ) binding by dried biomass of red, green and brown macroalgae [J]. Water Research, 2007, 41(4): 731-740.


