
Nanocrystalline Mg and Mg alloy powders by
hydriding-dehydriding processing
WANG Xin(王 辛), WANG Heng(王 珩), HU Lian-xi(胡连喜), WANG Er-de(王尔德)
School of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150001, China
Received 23 September 2009; accepted 30 January 2010
Abstract:
The process of mechanically assisted hydriding and subsequent thermal dehydriding was proposed to produce nanocrystalline Mg and Mg alloy powders using pure Mg and Mg-5.5%Zn-0.6%Zr (mass fraction) (ZK60 Mg) alloy as the starting materal. The hydriding was achieved by room-temperature reaction milling in hydrogen. The dehydriding was carried out by vacuum annealing of the as-milled powders. The microstructure and morphology of both the as-milled and subsequently dehydrided powders were characterized by X-ray diffraction analysis (XRD), transmission electron microscopy (TEM), and scanning electron microscopy (SEM), respectively. The results show that, by reaction milling in hydrogen, both Mg and ZK60 Mg alloy can be fully hydrided to form nanocrystalline MgH2 with an average grain size of 10 nm. After subsequent thermal dehydriding at 300 ?C, the MgH2 can be turned into Mg again, and the newly formed Mg grains are nanocrystallines, with an average grain size of 25 nm.
Key words:
Mg; Mg alloy; hydriding; dehydriching; hydrogen treatment; nanocrystalline;
1 Introduction
Because of their low density and high specific properties, Mg and Mg alloys are gaining increasing importance for applications including aerospace, automotive, materials handling, and portable electronic appliances[1-5]. However, Mg and Mg alloys usually suffer from relatively poor mechanical strength and are rarely used for high-performance structural applications[6-8]. In order to exploit their potential for such applications, the improvement of mechanical strength is urgently demanded.
It is well-known that grain refining is a general way to improve the mechanical strength of metallic materials. In most cases, the yield stress σy can be related to the grain size d by the Hall-Petch expression σy=σ0+kyd-1/2, where σ0 and ky are positive constants. For Mg alloys, the strengthening due to grain refining can be very tempting because of their high ky values[1, 9]. For example, when the grain size is reduced to 100-200 nm, the Mg97Zn1Y2 (molar ratio) alloy presents a yield strength as high as 610 MPa[10-11].
Powder metallurgy (P/M) is a quite potential way to prepare nanocrystalline or ultrafine grained bulk alloys. For most Mg alloys, however, it is difficult to produce nanocrystalline alloy powders by means of rapid solidification[12-15]. In an effort to establish an alternative way to produce nanocrystalline Mg and Mg alloy powders, we have developed the mechanically assisted hydriding-dehydriding process[16]. The present work reports an experimental investigation on the preparation of nanocrystalline Mg and ZK60 Mg alloy powders by using this new process.
2 Experimental
The commercially available pure Mg (≥99.5%, mass fraction) powders and as-cast ZK60 Mg alloy were used as the starting materials. The nominal composition of the as-cast ZK60 Mg alloy is shown in Table 1. Before being used for hydriding processing, the as-cast ZK60 Mg alloy was annealed at 450 ?C for 5 h and then crushed to powders by using a mechanical method. The hydriding was performed by mechanical milling in hydrogen at room temperature. The milling device was a planetary type QM-DY4, and the milling vial was made of stainless steel. For each batch of milling operation, 5 g of powders was loaded, and the mass ratio of ball to powder was 60?1. During milling, the mill shaft rotation was 400 r/min, the hydrogen pressure in the vial was kept above 0.5 MPa, and the pressure change was in-situ monitored for calculating the hydrogen absorbed by the alloy powders.
Table 1 Nominal composition of ZK60 alloy (mass fraction, %)

The dehydriding of the as-hydrided Mg and ZK60 Mg alloy powders was performed by using a home-made apparatus consisting of a closed volume set up with digital vacuum/pressure gauges, an electric heating and temperature monitoring system, and a vacuum pumping system. The mass of the powder sample for each desorption treatment was (0.500±0.005) g. The dehydriding was carried out at 300 ?C, and the hydrogen was desorbed from the sample at a constant test temperature in the initially evacuated volume of 1×10-2 Pa. The morphology of the powder samples before and after dehydriding treatment was observed by SEM, and the microstructure change was characterized by XRD and TEM, respectively.
3 Results and discussion
Fig.1 shows the hydrogenation kinetics curves of Mg and ZK60 Mg alloy by room-temperature mechanical milling in hydrogen. It was seen that the hydrogenation of both Mg and ZK60 Mg alloy was featured by a three-stage kinetics, i.e. slow-fast- saturation, as seen in our earlier study[17]. In the starting stage, the alloy absorbed hydrogen very slowly, which may be explained by the formation of Mg(H) solid solution (α phase) as a result of H adsorption and diffusion into the Mg lattice since powder surface and lattice defects were gradually developed in the as-milled powders with increasing milling time. After that, due to the initiation of the Mg hydriding reaction, that is, the transition of Mg(H) (α phase) to MgH2 (β phase), the hydrogen absorption kinetics was apparently accelerated, and this was seen to continue until the almost completion of the transition of α phase to β phase. In the final stage, the hydrogen absorption kinetics was again very slow, because the hydriding reaction was almost completed and gradually the hydrogen in the crystal lattice was saturated. Furthermore, it was found that the milling time to achieve full hydrogenation for Mg and ZK60 Mg alloy was about 16 h and 12 h, respectively, suggesting that the hydrogenation kinetics of ZK60 Mg alloy was faster than that of Mg. This can be attributed to alloying element Zr in the ZK60 Mg alloy, which is more affinitive to hydrogen. However, the alloying element Zn in the ZK60 alloy, which cannot absorb hydrogen, has a negative effect on the final hydrogen content achieved upon complete hydrogenation. Therefore, in the present study, the maximum hydrogen content achieved upon full hydrogenation in Mg was higher than in ZK60 Mg alloy, with the former being about 6.9% and the latter about 6.6% (mass fraction), respectively.
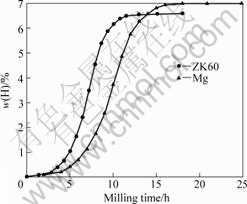
Fig.1 Hydrogenation kinetics curves of Mg and ZK60 Mg alloy during milling in hydrogen
Fig.2 shows the hydrogen desorption kinetics curves of the as-hydrided Mg and ZK60 Mg alloy powders during vacuum dehydriding at 300 ?C. The desorption kinetics of the as-hydrided ZK60 alloy was found to be faster than that of the as-hydrided Mg. It may be attributed to the possible substitution of some alloying element Zr or Zn to the Mg atoms in the MgH2 lattice, so that the MgH2 phase is to some extent destabilized. However, this needs to be further studied. By hydrogen discharge measurement, the time needed to achieve complete dehydriding upon vacuum annealing at 300 ?C was found to be about 120 min for Mg, and 80 min for ZK60 Mg alloy.
Fig.3 shows the XRD patterns of the starting, the mechanically hydrided, and the subsequently thermally dehydrided Mg and ZK60 Mg alloy powders. For both
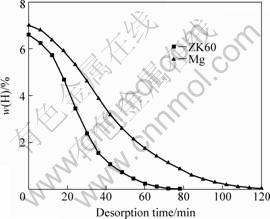
Fig.2 Desorption kinetics curves of as-hydrided Mg and ZK60 Mg alloy at 300 ?C
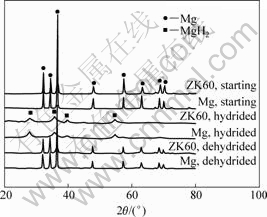
Fig.3 XRD patterns of starting, as-hydrided and subsequently dehydrided Mg and ZK60 Mg alloy
Mg and ZK60 Mg alloys, the XRD patterns were well fit with the standard diffraction peaks of Mg, but the peaks of ZK60 Mg alloy were a bit shifted to larger angles. This suggests that the ZK60 Mg alloy has a single Mg-based solid solution structure, and the major alloying element Zn in ZK60 Mg alloy does reduce the Mg lattice parameters by forming solid solution, as addressed by LIANG et al[18-19]. In comparison, after full hydrogenation by mechanical milling in hydrogen, no Mg diffraction peaks could be observed, except for those of the newly formed MgH2 phase. This indicates that the Mg phase has been fully hydrided by the mechanically driven solid-gas reaction:
Mg+H2→MgH2 (1)
Since the above reaction is reversible, the MgH2 phase can be turned into Mg after dehydriding treatment. As shown in Fig.3, the XRD patterns of the dehydrided Mg and ZK60 Mg alloy were again well fit with the standard diffraction peaks of Mg. By calculation based on the half width of the XRD peaks, the average grain size of MgH2 formed by mechanical milling of Mg and ZK60 Mg alloy in hydrogen was estimated to be about 10 nm, while that of the newly formed Mg phase upon full dehydriding at 300 ?C was about 25 nm. Though the grain size of the Mg phase after dehydriding treatment was to some extent coarser than that of the precursor MgH2 phase, it was still typically nanocrystalline and much smaller than the average grain size of 85 nm for the AZ31 Mg alloy obtained by mechanical milling in argon for 100 h[20]. That is to say, by the mechanically assisted hydrding-dehydriding process, microstructure nanocrystallization can be easily achieved in Mg and Mg alloy.
Fig.4 shows TEM images and the corresponding
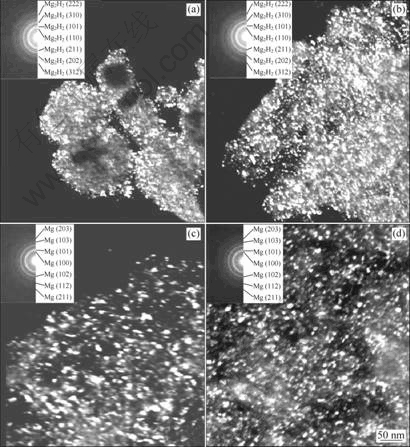
Fig.4 TEM images and diffraction patterns of as-hydrided and subsequently dehydrided Mg and ZK60 Mg alloy: (a) As-hydrided Mg; (b) As-hydrided ZK60 alloy; (c) Dehydrided Mg; (d) Dehydrided ZK60 alloy
electron diffraction patterns of both the as-hydrided and the subsequently dehydrided Mg and ZK60 Mg alloy, respectively. In the as-hydrided state (Figs.4(a) and (b)), MgH2 was identified to be the absolutely dominant phase, and the average grain size was estimated to be 10 nm. After dehydriding treatment at 300 ?C, complete dehydriding was achieved with the formation of a single Mg phase (Figs.4(c) and (d)). By TEM observation, most grains of the Mg phase obtained upon complete dehydriding were estimated to be 20-30 nm in size. Obviously, TEM observations agree well with the XRD results.
Fig.5 shows SEM morphologies of the starting, the as-hydrided, and the subsequently dehydrided Mg and ZK60 Mg alloy powders. The starting Mg and ZK60 Mg alloy powders were coarse particles with their average size above 100 μm (Figs.5(a) and (d)). After hydriding by mechanical milling in hydrogen, the particle size was greatly reduced. The as-hydrided Mg and ZK60 Mg alloy powders were featured by individual submicron-sized particles with a size of 0.5-1 μm and some agglomerates consisting of such submicron-sized particles (Figs.5(b) and (e)). After full dehydriding, though it seemed that a small fraction of individual submicron-sized particles agglomerated to form larger composite particles, little change could be observed in both the morphology and the size for most powders (Figs.5(c) and (f)). This suggests that, in general, the thermal dehydriding process carried out in the present study will not lead to sintering or severe agglomeration of the powder particles.
4 Conclusions
1) Nanocrystalline Mg and ZK60 Mg alloy powders can be produced by the mechanically assisted hydriding and subsequent thermal-dehydriding process.
2) The hydrogenation process of Mg and Mg alloy during mechanical milling in hydrogen is featured by a three-stage, i.e. “slow-fast-saturation”, kinetics, which can be attributed to the development of a Mg(H) solid solution, the transformation of Mg(H) to MgH2, and the formation of a full MgH2 microstructure, respectively.
3) The mechanically assisted fully-hydrided Mg and ZK60 Mg alloy powders are featured by a single MgH2 phase microstructure with an average grain size of 10 nm. By subsequent vacuum dehydriding at 300 ?C, the nano-structured MgH2 can be turned to nanocrystalline Mg phase with an average grain size of 25 nm.
4) By mechanically assisted hydriding, the particle size of Mg and Mg alloy powders can be greatly reduced. In particular, the as-hydrided Mg and ZK60 Mg alloy powders obtained were 0.5-1 μm in average size, and sintering or severe agglomeration of the powder particles was not observed during thermal dehydriding.

Fig.5 Morphologies of starting, as-hydrided and subsequently dehydrided Mg and ZK60 Mg alloy powders: (a) Starting Mg; (b) As-hydrided Mg; (c) Dehydrided Mg; (d) Starting ZK60 alloy; (e) As-hydrided ZK60 alloy; (f) Dehydrided ZK60 alloy
References
[1] Chen Zhen-hua, Yan Hong-ge, Chen Ji-hua, QUAN Ya-jie, WANG Hui-min, CHEN Ding. Magnesium alloy [M]. Beijing: Chemical Industry Press, 2004: 1-10. (in Chinese)
[2] Mordike B L, Ebert T. Magnesium: properties—applications— potential [J]. Materials Science and Engineering A, 2001, 302(1): 37-45.
[3] Pekguleryuz M O, Kaya A A. Creep resistant magnesium alloys for powertrain applications [J]. Advanced Engineering Materials, 2003, 5(12): 866-878.
[4] Mehta D S, Masood S H, Song W Q. Investigation of wear properties of magnesium and aluminum alloys for automotive applications [J]. Journal of Materials Processing Technology, 2004, 155/156: 1526-1531.
[5] YU Kun, RUI Shou-tai, WANG Xiao-yan, WANG Ri-chu, Li Wen-xian. Texture evolution of extruded AZ31 magnesium alloy sheets [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(3): 511-516.
[6] Qian M, Das A. Grain refinement of magnesium alloys by zirconium: Formation of equiaxed grains [J]. Scripta Materialia, 2006, 54(5): 881-886.
[7] Liang Shu-jin, Liu Zu-yan, Wang Er-de. Microstructure and mechanical properties of Mg-Al-Zn alloy deformed by cold extrusion [J]. Materials Letters, 2008, 62(17/18): 3051-3054.
[8] WANG Bin, YANG Yuan-sheng, ZHOU Ji-xue, TONG Wen-hui. Microstructure refinement of AZ91D alloy solidified with pulsed magnetic field [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(3): 536-540.
[9] Chang T C, Wang J Y, O C M, Lee S. Grain refining of magnesium alloy AZ31 by rolling [J]. Journal of Materials Processing Technology, 2003, 140(1/3): 588-591.
[10] Akihisa I, Mitsuhide M, Yoshihito K, Kenji A, Kentaro H, Junich K. Novel hexagonal structure of ultra-high strength magnesium-based alloys [J]. Materials Transactions, 2002, 43(3): 580-584.
[11] Akihisa I, Yoshihito K, Mitsuhide M, Kentaro H, Junichi K. Novel hexagonal structure and ultrahigh strength of magnesium solid solution in the Mg-Zn-Y system [J]. Journal of Materials Research, 2001, 16(7): 1894-1900.
[12] GOVIND K, Suseelan N, Mittal M C, Lal K, Mahanti R K, Sivaramakrishnan C S. Development of rapidly solidified (RS) magnesium-aluminium-zinc alloy [J]. Materials Science and Engineering A, 2001, 304//305/306: 520-523.
[13] Sheng Shao-ding, Chen Ding, Chen Zhen-hua. Effects of Si addition on microstructure and mechanical properties of RS/PM (rapid solidification and powder metallurgy) AZ91 alloy [J]. Journal of Alloys and Compounds, 2009, 470(1/2): L17-L20.
[14] Cai Jing, Ma Guo-hong, Liu Zheng, Zhang Hai-feng, Wang Ai-min, HU Zhuang-qi. Influence of rapid solidification on the mechanical properties of Mg-Zn-Ce-Ag magnesium alloy [J]. Materials Science and Engineering A, 2007, 456(1/2): 364-367.
[15] Czerwinski F. Magnesium alloy particulates for thixomolding applications manufactured by rapid solidification [J]. Materials Science and Engineering A, 2004, 367(1/2): 261-271.
[16] HU Lian-xi, Wu Yang, Yuan Yuan, Wang Heng. Microstructure nanocrystallization of a Mg-3wt.%Al-1wt.%Zn alloy by mechanically assisted hydriding-dehydriding [J]. Materials Letters, 2008, 62(17/18): 2984-2987.
[17] Wang Heng, Wang Xin, Hu Lian-xi. Preparation of nanocrystalline MgH2 by solid-gas milling in hydrogen [J]. Rare Metal Materials and Engineering, 2009, 38 (4): 696-699. (in Chinese)
[18] Liang G X. Synthesis and hydrogen storage properties of Mg-based alloys [J]. Journal of Alloys and Compounds, 2004, 370(1/2): 123-128.
[19] LIANG G X, Schulz R. Synthesis of binary Mg-based alloys by mechanical alloying [J]. Journal of Metastable and Nanocrystalline Materials, 2002, 12: 93-110.
[20] WANG Heng, HU Lian-xi, CHEN Xian-jue, WANG Er-de. Preparation of nanocrystalline magnesium alloy powders by high-energy ball milling [J]. Powder Metallurgy Technology, 2008, 26(6): 403-406. (in Chinese)
(Edited by YANG Bing)
Foundation item: Project(50574034) supported by the National Natural Science Foundation of China; Project(20060213016) supported by Doctoral Education Fund of Ministry of Education of China
Corresponding author: HU Lian-xi; Tel: +86-451-86418613; E-mail: hulx@hit.edu.cn
DOI: 10.1016/S1003-6326(09)60299-5


