
Diffusion bonding of Ti-6Al-4V by pulse current heating and hot-pressing
HE Dai-hua(何代华), FU Zheng-yi(傅正义), WANG Wei-min(王为民)
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing,
Wuhan University of Technology, Wuhan 430070, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
Pulse current heating (PCH) was used to join Ti-6Al-4V alloy at two cases of with die and without die. Hot-pressing (HP) method was used to provide a comparison between the two methods. Microstructures near the contacting surfaces were observed in optical microscope. Temperature distribution was analyzed. After joining, the tensile properties of the samples were evaluated. Experimental results show that grains and phases grow transversely on contacting surfaces, which makes two parts into a whole with a certain of tensile strength. PCH joining is a different temperature joining method. The highest temperature is located at the interface. The comparison of tensile strengths of samples joined by the two methods indicates that joining temperature and holding time needed by PCH are lower and less than those needed for HP.
Key words:
pulse current heating; Ti-6Al-4V; joining; hot-pressing; temperature distribution;
1 Introduction
Ti-6Al-4V, an α+β beta alloy, is an important alloy, which has been widely used for various structures in the aerospace and marine industries. It is being used as a potential candidated material for critical components in satellite and launch vehicles at very high stress levels due to its excellent corrosion resistance, and a good combination of strength and toughness and outstanding strength-to-mass ratios. The requirement of sound and reliable joins in these components is imperative.
Some methods, such as tungsten-inert gas (TIG) welding, plasma arc welding and electron beam welding, were usually used to join Ti-6Al-4V alloy[1]. But the processes are complicated and there are problems concerning efficiency or properties. Some of the processes are processed at about 900 ℃ or above, so joined samples need a postweld heat treatment to improve the microstructures and properties[2]. And the affinity of titanium for air and other gases increases at high temperature, so it is very critical to shield the vicinity of the join zone from air completely.
Spark plasma sintering (SPS), a non-conventional sintering technique, was developed in Japan[3]. It enjoys certain inherent advantages, such as: high thermo-efficiency; quick heating-up; better self- purification of the surface of particle and sintering activity; and fast sintering at low temperatures[3]. There are many reports on the fabrication of ceramics by this technique (oxides, SiC, AlN, Cermets, etc)[4-8]. Except sintering, similar process was also made in joining of metals, ceramics or intermetallics[9] or crystallization of bulk metallic glasses [10]. In SPS process, a pulse direct current is allowed to pass through electrically conducting die and in appropriate cases. This implies that the die also acts as a heating source and that sample is heated from both outside and inside. In this respect, the authors suggested that the process called pulse current heating (PCH) might be more suitable.
2 Experimental
Annealing Ti-6Al-4V alloy was used as the experimental material. The composition and properties are shown in Table 1.
The experimental steps were as follows: (1) Titanium plates with the size of 20 mm×20 mm were obtained from the Ti-6Al-4V stick; (2) Surfaces of the plates were polished and cleaned thoroughly with emery papers; (3) Plates were pickled and etched by etchants with following chemical composition: 30%HNO3+4% HF+66%H2O(volume fraction)[11], then degreased with acetone prior to joining[12].
Table 1 Chemical composition and properties of Ti-6Al-4V alloy (commercial data)

In the PCH apparatus, two plates to be joined were introduced under different arrangements. In one case, the plates were situated between punches without the die and in the other case a die was used, as in conventional SPS experiments. In both cases a pressure of 5 MPa was applied. For the first case joining was investigated at 600, 650 and 700 ℃, with a holding time of 5 min under an atmosphere of high purity argon (purity 99.99 %). In the second case, the selected temperatures were 750, 800, and 850 ℃ and the holding time was 10 min.
Experiments were also conducted in a hot-pressing (HP) to provide a comparison between the two methods. Joining was investigated at 800 ℃ with holding times of 30 and 60 min under a pressure of 16 MPa and an argon atmosphere. In this case no graphite die was used.
Fig.1 shows the schematic diagrams of the PCH technique with and without the graphite die. Fig.2 shows the schematic diagram of HP technique.
After joining, microstructures were evaluated by optical microscopy. Temperature distribution was analyzed. The tensile properties of the samples were evaluated. Every reported tensile strength value was the average of at least three specimens for every joining condition. The tensile strength of the joined Ti-6Al-4V alloys was determined.
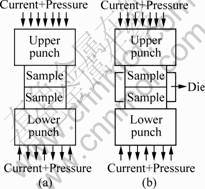
Fig.1 Schematic diagrams of joining by PCH: (a) Without graphite die; (b) With graphite die
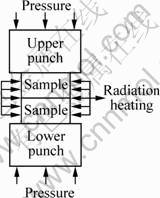
Fig.2 Schematic diagram of joining by HP
3 Results and discussion
3.1 Microstructures
The microstructures near the interface of samples joined by PCH are shown in Fig.3, and Fig.4 shows the strip-shaped α and β beta matrix. Fig.3 shows the microstructure of the area near a joint made at 700 ℃ without a die and Fig.4 shows the corresponding microstructure for a joint made at 850 ℃ with a graphite die. In both cases, the general features of the microstructure are relatively similar. As can be seen from Figs.3 and 4, the microstructural features cross the interface, indicating the formation of a good joint.
The microstructures near the interface of samples joined by HP shown in Fig.5, indicate that the contacting surfaces are clear, there are less grains and phases grow transversely on the surfaces.
3.2 Temperature distribution
Fig.6 shows the photographs during 700 ℃ joining temperature with a holding time of 10 min under 5 MPa pressure. Figs.6(a) and (b) are the initial moment and the 5th minute of preserving-heat stage, respectively. The results clearly show that the temperature distribution is not uniform with the highest value at the interface indicated by different colors. The temperature decreases in both directions away from the interface towards the opposite ends of the plates. The fact that the highest temperature is located at the interface not only lends itself to the feasibility of joining but also ensures that the rest of the sample is not subjected to high temperatures, and thus minimizing the influence of high temperature on the properties of the samples. Figs.6(a) and (b) show no significant change in the distribution pattern, indicating that a steady state distribution is achieved at the initial moment of preserving-heat stage. And the highest temperature is obtained at the interface. In this case, a steady state distribution is reached at the initial moment of preserving-heat stage.
In a PCH process, samples are heated directly by pulse direct current. It is also to say that the samples are heated by Joule heat. The heat Q can be expressed as follows:
Q=I2Rt (1)
where I, R and t are current, contacting resistivity and time, respectively.
R is larger for that only some points or little areas connected between two joining surfaces at the initial stage. So Q of the contacting surface is larger, and the temperature here is higher than that of the opposite ends of the plates. Furthermore, the opposite ends of the plates are cooled by water. From the above results, it can be obtained that the PCH joining is a different temperature joining.
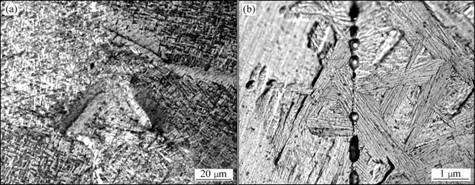
Fig.3 Microstructures of sample joined by PCH at 700 ℃, 5 MPa and 5 min without graphite die
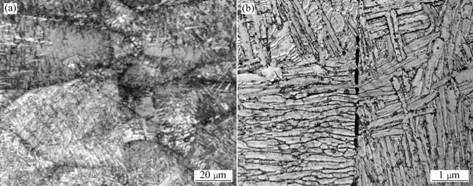
Fig.4 Microstructures of sample joined by PCH at 850 ℃, 5 MPa and 10 min with graphite die
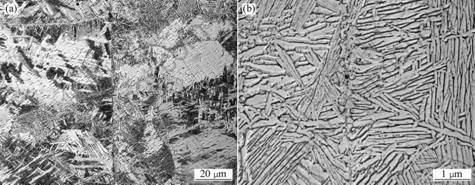
Fig.5 Microstructures of the sample joined by HP at 800 ℃, 5 MPa and 10 min without graphite die
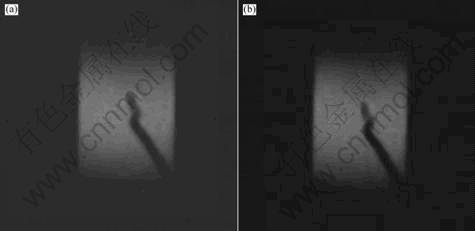
Fig.6 Photos of sample joined at 700 ℃, 5 MPa pressure and 10 min holding time: (a) Initial moment of preserving-heat stage; (b) The 5th minute of preserving-heat stage
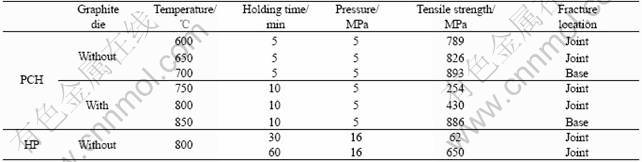
Table 2 Comparison of samples under different conditions
3.3 tensile strength
Room temperature tensile strength and the fracture location of the samples joined under different conditions are shown in Table 2.
For samples joined by the PCH method, the tensile strength shows a dependence on the joining temperature, and increases with the increase of temperature for both cases where a die is used or not used. The tensile strength for samples joined with a die is systematically lower than those obtained with a die for corresponding temperatures. It is due to the different current densities to the two cases. In the “no die” case, the current density is higher than that in the “with die” case. And the higher current density has enhanced mass transport.
The temperature has also an effect on the location of failure. Not only the joints are stronger when they are formed at higher temperature, but also the samples fail in the base alloy and not in the joint for both cases where a die is used or not used, as can be seen from Table 2. These results confirm that good joints are made under these conditions.
In contrast to the above, joints made by the HP method are not satisfactory even when bonding is attempted at 800 ℃ with a higher pressure (16 MPa) and extended holding time(60 min).
It is important to point out that both the temperature and holding time needed by the PCH method to affect good bonding are lower and shorter, respectively, than those of the superplastic formation/diffusion bonding (SPF/DB)[13].
The advantage of the PCH method stems from the uneven distribution of temperature that is a consequence of non-uniform resitistivities. The interface has a higher contact resistance that results in the higher local temperatures[14]. Thus in addition to the high rate of heating by current, the uneven temperature distribution is a major benefit of this technique. It should also be pointed out that the current can play an additional role besides providing Joule heating. Recent investigations have demonstrated the role of the current in the enhancement of mass transport[15-20].
4 Conclusions
1) The microstructures near joint of the sample joined by PCH show a mixture of a large of serrated and acicular alpha with retained β matrix. The grains and phases grow transversely on contacting surfaces.
2) PCH joining is a different temperature method. The highest temperature located at the interface not only lends itself to the feasibility of joining but also ensures that the rest of the sample is not subjected to high temperatures, and thus minimizing the influence of high temperature on the properties of the samples.
3) Ti-6Al-4V alloy joined structure with high tensile strength can be fabricated by PCH method at 700 ℃ with 5 min holding time and 5 MPa pressure in the case without die. At 800 ℃ joining temperature, 16 MPa pressure with holding times of 30 and 60 min, the tensile strengths of the samples joined by HP are only 62 MPa and 650 MPa. PCH method is more effective than HP to join. To Ti-6Al-4V alloy joining, the case without die is optimal to that with die.
Acknowledgement
The authors wish to express their gratitude to YAN You-wei, State Key Laboratory of Die Technology, Huazhong University of Science and Technology for his help.
References
[1] YOKOTA K, SASANO R, KAWJIRI I. Fundamental study on electron beam welding of heavy thickness 6Al-4V titanium alloy[J]. Joining in the World, 1988, 26(9/10): 202-215.
[2] KESHAVA K, MURTHY, SUNDARESAN S. Fracture toughness of Ti-6Al-4V after welding and postweld heat treatment[J]. Joining Journal, 1997, 76(2): 81-91.
[3] Tokita M. Trends in advanced SPS spark plasma sintering systems and technology[J]. J Soc Powder Technol Jpn, 1993, 30(11): 790-804.
[4] GAO L, SHEN Z J, MIYAMOTO H, NYGREN M. Superfast densification of oxide/oxide ceramic composites[J]. J Am Ceram Soc, 1999, 82(4): 1061-1063.
[5] ZHOU Y, HIRAO K, TORIYAMA M, TANAKA H. Very rapid densification of nanometer silicon carbide powder by pulse electric current sintering[J]. J Am Ceram Soc, 2000, 83(3): 654-656.
[6] JAYASEELAN D D, RANI D A, NISHIKAWA T, AWAJI H, OHJI T. Sintering and microstructure of mullite/mo composites[J]. J Eur Ceram Soc, 2002, 22(7): 1113-1117.
[7] FU Zheng-yi, LIU Jun-fang, WANG Hao, HE Dai-hua, ZHANG Qing-jie. Spark plasma sintering of aluminum nitride transparent ceramics[J]. Materials Science and Technology, 2004, 20(9): 1097-1099.
[8] WANG Yu-cheng, FU Zheng-yi. Study temperature field in spark plasma sintering[J]. Materials Science and Engineering B, 2002, 90: 34-37.
[9] LIU Wei-ping, MASAAKI N. In situ joining of dissimilar nanocrystalline materials by spark plasma sintering[J]. Scripta Materialia, 2003, 48(9): 1225-1230.
[10] HOLLAND T B, L?FFLER J F, MUNIR Z A. Crystallization of metallic glasses under the influence of high density DC currents[J]. J Applied Physics, 2004, 95(5): 2896-2899.
[11] WOOLCOCK A. The effect of welding speed and edge preparation on the incidence of porosity in TIG welding titanium[C]// WILLIAM J O, BELOV A F, eds. Titanium and Titanium Alloys, Scientific and Technological Aspects. Vol 2. Plenum Press, 1982: 118.
[12] THOMAS G, RAMACHANDRA V, NAIR M J, NAGARAJAN K V. Effect of preweld and postweld heat treatment on the properties of gta welds in Ti-6Al-4V sheet[J]. Welding Journal, 1992, 71(1): 15s-20s.
[13] ISLAM M F, PILLING J, RIDLEY N. Effect of surface finish and sheet thickness on isostatic diffusion bonding of superplastic Ti-6Al-4V[J]. Mater Sci Techno, 1997, 13(12): 1045-1050.
[14] ZHANG Dong-ming, FU Zheng-yi, YUAN Run-zhang, et al. Equivalent resistance in pulse electric current sintering[J]. Journal of Wuhan University of Technology, 2002, 17(2): 30-32.
[15] FRIEDMAN J R, GARAY J E, ANSELMI-TAMBURINI U, MUNIR Z R. Modified interfacial reactions in Ag-Zn multilayers under the influence of high DC currents[J]. Intermetallics, 2004, 12: 589.
[16] GARAY E, ANSELMI-TAMBURINI U, MUNIR Z A. Enhanced growth of intermetallic phases in the Ni-Ti system by current effects[J]. Acta Mater, 2003, 51: 4487.
[17] BERTOLINO N, GARAY J, ANSELMI-TAMBURINI U, MUNIR Z R. High-flux current effects in interfacial reactions in Au-Al multilayers[J]. Phil Mag B, 2002, 82: 969.
[18] HUNTINGTON H B. Diffusion in Solids[M]. NOWICK A S, BURTON J J. New York: Academic Press, 1975: 306.
[19] ASOKA-KUMAR P, ALATALO M, GOSH V J, KRUSEMAN A C, NIELSON B, LYNN K G. Increased elemental specificity of positron annihilation spectra[J]. Phys Rev Lett, 1996, 77: 2097.
[20] GARAY J E, GLADE S C, ANSELMI-TAMBURINI U, ASOKA-KUIMAR P, MUNIR Z A. Electric current enhanced defect mobility in Ni3Ti intermetallics[J]. Appl Phys Lett, 2004, 85: 573.
(Edited by CHEN Can-hua)
Foundation item: Project(50272047) supported by the National Natural Science Foundation of China
Corresponding author: HE Dai-hua; E-mail: hedh21@163.com


