Trans. Nonferrous Met. Soc. China 29(2019) 1503-1509
Fabrication and magnetic transformation from paramagnetic to ferrimagnetic of ZnFe2O4 hollow spheres
Yi-cheng GE, Zhi-long WANG, Mao-zhong YI, Li-ping RAN
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 26 October 2018; accepted 12 May 2019
Abstract:
ZnFe2O4 hollow spheres (ZFHs) with sizes of 200-302 nm were synthesized by simple impregnating method using the as-prepared phenolic formaldehyde (PF) spheres as templates and subsequent annealing at 500-700 °C. The prepared ZFHs are assembled by a large number of small nanoparticles with sizes of 15-20 nm, and many mesopores exist among these nanoparticles. The samples annealed at 500-550 °C exhibit a single cubic spinel structure, while higher annealing temperature leads to the formation of hexagonal ZnO and rhombohedral α-Fe2O3 secondary phases. The size of the assembled nanoparticles increases with the increase in annealing temperature. Novel magnetic transformation from paramagnetic to ferrimagnetic is induced by the reduction of annealing temperature and the saturation magnetization significantly increases from 2.3 to 13.5 A·m2/kg. The effect of the formation of hollow sphere structure on the redistribution of Fe3+ and Zn2+ in the spinel structure was studied.
Key words:
ZnFe2O4 hollow spheres; nanoparticles; magnetic properties; magnetic transformation;
1 Introduction
Zinc ferrite (ZnFe2O4) nanomaterials have recently attracted considerable attention for potential applications in microwave absorption [1], energy storage [2] and drug delivery [3] because of their remarkable properties, such as high specific surface areas, excellent magnetic properties, and non-toxicity. Nanocrystalline ZnFe2O4 ferrites have spinel structures and the particle size has a significant effect on the permeability, saturation magneti- zation, and microwave adsorption performance [4,5]. When the ZnFe2O4 particle size is decreased from 110 to 15 nm, the magnetic behavior of the particles shifts from paramagnetic to ferrimagnetic, and the Ms increases from 5.0 to 7.0 A·m2/kg [6]. This enhanced magnetization originates from the super-exchange interaction-induced magnetic order, which can be attributed to the inversion of the Fe3+ and Zn2+ cations in the tetrahedral and octahedral crystalline structure positions [7]. ZnFe2O4 nanoparticles with ferrimagnetic properties have been prepared by physical and chemical methods such as traditional ceramic synthesis, aerogel procedure, and ball milling [7-9]. Meanwhile, ZnFe2O4 hollow nanospheres have also been reported to be fabricated by solvothermal process combined with the subsequent thermal treatment process. Some Zn-based ferrite hollow spheres, such as ZnFe2O4/graphene [10] and ZnFe2O4@SiO2 [11], are reported to exhibit excellent microwave absorption owing to their high saturation magnetization, suitable impedance and ability to cause multiple reflection and scattering of incident microwaves [10]. However, the transformation of ZnFe2O4 hollow spheres from paramagnetic to ferrimagnetic, which is found to significantly affect their magnetic properties, has not yet been reported.
In the present study, ZnFe2O4 hollow nanospheres were synthesized by solvothermal method using the phenolic formaldehyde (PF) spheres as templates and subsequent annealing to remove the PF templates. The crystalline structure, morphology, magnetic properties and microwave absorbing properties were investigated.
2 Experimental
2.1 Preparation of phenolic formaldehyde (PF) spheres
Phenolic formaldehyde (PF) spheres were prepared by the hydrothermal approach and used as hard templates. Typically, 0.50 g of resorcinol was added into a fresh mixture composed of 0.5 mL of NH3·H2O, 30 mL of C2H5OH, and 100 mL of deionized water to form a clear solution with strong stirring, and then 1.4 mL of CH2O was added and stirred for 12 h. Small PF nanoparticles were thereafter nucleated. After that, the solution was then transferred into a Teflon-lined autoclave and further polymerized to form PF spheres at 100 °C for 24 h. The final PF spheres (CmHn) were isolated via centrifugation and vacuum dried at 60 °C for 12 h.
2.2 Preparation of ZnFe2O4 hollow nanospheres
ZnFe2O4 hollow nanospheres were synthesized by simple one-pot solvothermal method using the PF spheres as templates. 0.5 g of the as-prepared PF templates were dispersed in 60 mL of mixed clear solution comprising of 2 mol/L Fe(NO3)3 and 1 mol/L Zn(NO3)2. After being ultrasonically dispersed for 1 h and aged for 3 h, Fe3+, Zn2+ and 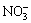 cations were absorbed on the surface of the PF spheres. Subsequently, the brown product was collected via centrifugation, carefully washed and dried in vacuum at 50 °C for 24 h. The PF core and residual
cations were absorbed on the surface of the PF spheres. Subsequently, the brown product was collected via centrifugation, carefully washed and dried in vacuum at 50 °C for 24 h. The PF core and residual 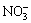 ions were then removed by calcination in muffle at 500-700 °C in air for 3 h to obtain ZnFe2O4 hollow spheres.
ions were then removed by calcination in muffle at 500-700 °C in air for 3 h to obtain ZnFe2O4 hollow spheres.
2.3 Characterization
The as-synthesized samples were characterized by X-ray diffraction (XRD, Rigaku, D/Max 2500, Cu Kα radiation), field-emission scanning electronic microscope (FESEM, Quanta FEG 250), Philips CM 200 transmission electron microscope (TEM), vibrating sample magnetometer (MLVSM9 MagLab 9 T, Oxford Instrument), and vector network analyzer (Agilent N5234A). Thermal stability of PF samples was analyzed via thermogravimetric analysis from 20 to 900 °C in air.
3 Results and discussion
3.1 Phenolic formaldehyde characterization
Phenolic formaldehyde (PF) templates were prepared by the hydrothermal method. The corresponding SEM image (Fig. 1(a)) reveals that the monodisperse PF templates are about 800 nm in size with a narrow size distribution. The smooth surface is critical for the subsequent absorption of Fe3+, Zn2+ and NO3- ions according to the electrostatic adsorption and coordination between atoms. The TG-DTA curve (Fig. 1(b)) reveals that these PF resins could be easily oxidized and removed by calcination at temperatures higher than 500 °C. During the calcination process, the absorbed Zn(NO3)2 and Fe(NO3)3 will be decomposed into ZnO and Fe2O3, and further transformed into ZnFe2O4. The chemical reaction can be expressed by the following formulas:
CmHn+O2→CO2+H2O (1)
Zn(NO3)2→ZnO+NO2↑+O2↑ (2)
Fe(NO3)3→Fe2O3+NO2↑+O2↑ (3)
ZnO+Fe2O3→ZnFe2O4 (4)
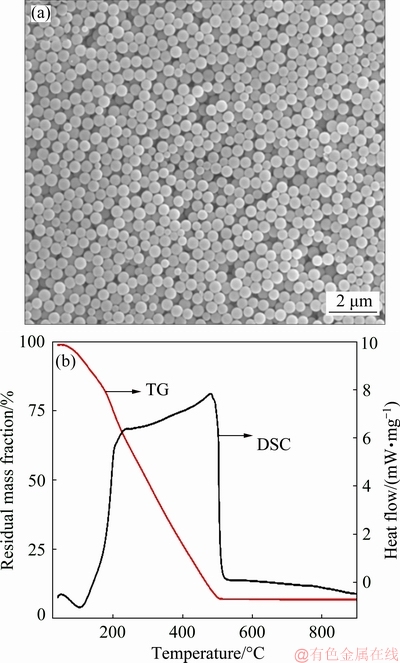
Fig. 1 SEM image (a) and TG-DTA curve (b) of PF templates
3.2 Crystal structure and chemical composition
As shown in Fig. 2, the XRD patterns of the samples calcined at 500-700 °C confirm the formation of ZnFe2O4 (JCPDS card No. 02-1043) with a cubic spinel structure and the depletion of amorphous carbon or any carbide phases, indicating the removal of PF templates. A small amount of hexagonal ZnO and rhombohedral α-Fe2O3 secondary phases is observed after calcination at 600-700 °C. It is supposed that this cubic spinel structure is much more stable after calcination at 500-550 °C and the sharp diffraction peaks also indicate that they are well-crystallized. The average crystallite size of ZnFe2O4 was determined by using Scherrer’s formula [12] from the width of the prominent peak (311) and the lattice parameter a was refined using Jade 5 software program. As depicted in Table 1, the average crystallite size and the lattice parameter increase with annealing temperature. This is similar with the results reported in Refs. [13,14]. Bulk ZnFe2O4 crystallizes in a normal cubic spinel structure with all the Fe3+ cations resided in the larger octahedral sites (B sites) and Zn2+ cations totally in the smaller tetrahedral sites (A sites). The reduction of the lattice parameter is an indication that a redistribution process of Fe3+ and Zn2+ cations occurs with the decrease of calcination temperature.
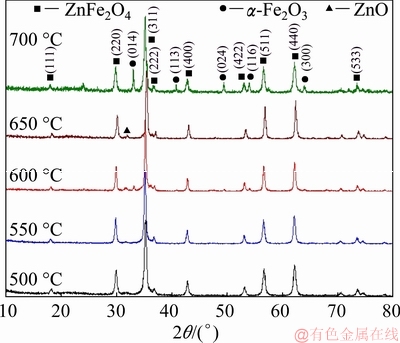
Fig. 2 XRD patterns of ZnFe2O4 hollow spheres calcined at 500-700 °C
3.3 Infrared spectrum characterization
The vibrations of the ions in the crystal lattice are usually detected by the absorption bands in 200-800 cm-1 infrared region. With respect to cubic ferrites, the absorption ν1 round 550 cm-1 is caused by the tetrahedral Fe—O stretching vibrations. The FTIR spectra of ZnFe2O4 (Fig. 3) reveal that stretching vibrations in tetrahedral site are detected round 544 cm-1 with 544.7, 544.5, 543.8, and 543.2 cm-1 for ZnFe2O4 annealed at 500, 550, 600 and 650 °C, respectively. This slight shift towards the lower wavenumber with the increase of annealing temperature indicates the changes of the crystallite size and the metal–oxygen bonds in ZnFe2O4 due to the transition from mixed spinel to normal structure. This is in agreement with the XRD results. In addition, the bands at 3443 and 1632 cm-1 correspond to the stretching and bending vibrations of hydroxyl, respectively. As compared with the standard bands (ν=3600-3650 cm-1), the hydroxyl absorption peak shifts towards the lower wavenumber and becomes strengthening and broadening, indicating that a large number of hydroxyl exists on the surface of ZnFe2O4 hollow spheres. Hydroxyl groups not only could increase the surface free energy and react with many kinds of compounds, but also could provide a foundation for self-assembly and protein functionalization. This characteristic is helpful for the carriage of medicine.
3.4 Microscopic morphology
Figure 4 shows the SEM images and the size distribution of ZnFe2O4 hollow spheres. The obtained spheres are mono-disperse and have crumpled surfaces in favor of large surface areas. The existence of broken spheres indicates that they are hollow in nature. As compared with the sizes of PF templates (800 nm), the diameters of ZnFe2O4 hollow spheres are significantly reduced to approximately 200-300 nm. This could be due to the volatilization of the PF templates and the densification and shrinkage of ZnFe2O4 during the heating process [18]. The sphere sizes for the samples annealed at 500, 550, 600 and 700 °C are about 230, 302, 280 and 200 nm, respectively. The size distribution (Figs. 3(c, f, i, l)) reveals that the hollow spheres are more uniform after calcination at temperatures lower than 550 °C. The sample obtained at 500 °C is uniform in both morphology and sphere size, and exhibits a very narrow size distribution. The inhomogeneous growth and agglomeration of the hollow spheres after calcination at 700 °C could be attributed to the extensive grain growth of ZnFe2O4 nanoparticles and the substantial shrinkage of ZnFe2O4 hollow spheres.
Table 1 Particle size, lattice parameter (a), saturation magnetization (Ms) and coercivity (Hc) value of ZnFe2O4 hollow spheres calcined at 500-700 °C
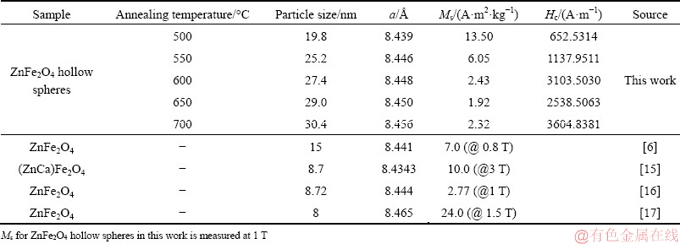

Fig. 3 FTIR spectra of ZnFe2O4 hollow spheres calcined at 500-700 °C
The sample calcined at 500 °C is further investigated by TEM, as shown in Fig. 5. It is found that they are fairly uniform in both morphology and size with the diameter centering at about 270 nm, which is in agreement with the SEM results. Figure 5(b) shows that the hollow spheres are assembled by a large number of small nanoparticles with sizes of 15-20 nm, and many mesopores exist among these nanoparticles. The contrast between the black edge and the bright center also confirms the hollow nature. Furthermore, the corresponding SAED pattern confirms that these nanoparticles are crystalline and have a cubic spinel structure. The high resolution TEM image in Fig. 5(d) shows an interplanar spacing of approximately 0.25 nm corresponding to (311) crystal plane of the cubic spinel ZnFe2O4 phase.
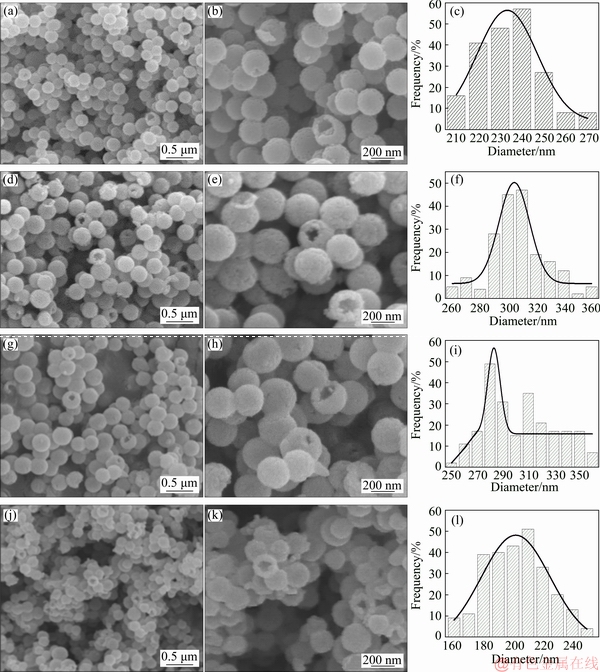
Fig. 4 SEM images and size distributions of ZnFe2O4 hollow spheres calcined at 500 °C (a-c), 550 °C (d-f), 600 °C (g-i) and 700 °C (j-l)

Fig. 5 TEM bright field images (a, b), SAED pattern (c) and HRTEM image (d) for ZnFe2O4 hollow spheres calcined at 500 °C
3.5 Magnetic properties
The magnetization curves of the ZnFe2O4 hollow spheres measured at a maximum magnetic field of 1 T are shown in Fig. 6. The corresponding coercivity (Hc) and the saturation magnetization (Ms) are listed in Table 1. The magnetization curves show a presence of magnetic behavior transformation from paramagnetic to ferrimagnetic with M-H loops changing from a straight line with a positive slope for those annealed at 600-700 °C to a typical S-type shape for the samples annealed at 500-550 °C. Accordingly, the samples annealed at 600-700 °C exhibit relatively low Ms values of 1.92-2.31 A·m2/kg and large Hc of 2538.5063- 3604.8381 A/m, while those annealed at 500-550 °C show high Ms of 6.05-13.50 A·m2/kg and low Hc of 652.5314-1137.9511 A/m. The linear increase of Hc with temperature also indicates a higher degree of disorder in the magnetic moment arrangement at higher calcination temperatures. The slight increase in Ms with the increase of temperature from 650 to 700 °C can be attributed to the formation of minor paramagnetic rhombohedral α-Fe2O3 phase. The magnetic transaction behavior is found to be in great agreement with that reported for the ZnFe2O4 nanoparticles in Refs. [6,15-17].
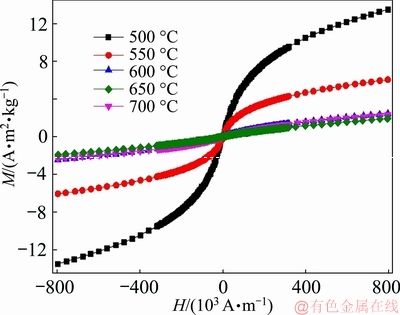
Fig. 6 M-H loops of ZnFe2O4 hollow spheres measured at maximum magnetic field of 1T
The observed magnetic transformation for the ZnFe2O4 hollow spheres can be ascribed to the redistribution of Fe3+ and Zn2+ in the spinel structure, as shown by the XRD and FTIR results. ZnFe2O4 hollow spheres calcined at 600-700 °C are of a normal spinel with diamagnetic Zn2+ cations at the A sites and magnetic Fe3+ ions occupying B sites. The strong intra-sublattice (A-A and B-B) interactions and the absence of inter-sublattice (A-B) superexchange interaction result in a strong paramagnetic characteristic [19]. However, ZnFe2O4 hollow spheres calcined at 500-550 °C exhibit partially inverse spinel structure, where Zn2+ and Fe3+ distribute over A and B sites. The stronger A-B superexchange interaction as compared to A-A and B-B interactions is responsible for the novel magnetic properties such as nonzero net magnetic moment and ferrimagnetism at room temperature. In addition, as compared to ZnFe2O4 nanoparticles reported in Refs. [6,15,16] as shown in Table 1, the as-prepared hollow spheres calcined at 500 °C exhibit much higher Ms, extensively lower coercivity, and slightly larger nanoparticle size. It is reasonable to deduce that large inversion parameters could be obtained by the formation of hollow sphere structure. The mesoporous ZnFe2O4 hollow spheres with ferrimagnetic characteristic obtained in this study may be used in microwave absorption and drug delivery.
4 Conclusions
(1) ZnFe2O4 hollow spheres with a diameter of 200-302 nm are successfully fabricated by solvothermal method with the use of phenolic formaldehyde (PF) sphere templates and subsequent annealing. The hollow spheres have the crumpled shell assembled by many nanoparticles with sizes of 15-20 nm. The size of the assembled nanoparticles increases with the increase in annealing temperature.
(2) The ZnFe2O4 hollow spheres annealed at 500-550 °C exhibit a single cubic spinel structure, while higher annealing temperature leads to the formation of hexagonal ZnO and rhombohedral α-Fe2O3 secondary phases.
(3) Novel magnetic transformation from paramagnetic to ferrimagnetic is confirmed by the reduction of annealing temperature and the saturation magnetization significantly increases from 2.3 to 13.5 A·m2/kg. The formation of hollow sphere structure benefits to the redistribution of Fe3+ and Zn2+ in the spinel structure.
References
[1] YAN Ai-guo, LIU Xiao-he, YI Ran, SHI Rong-rong, ZHANG Ning, QIU Guang-zhou. Selective synthesis and properties of monodisperse Zn ferrite hollow nanospheres and nanosheets [J]. Journal of Physical Chemistry C, 2008, 112(23): 8558-8563.
[2] JIN Ren-cheng, JIANG Hua, SUN Ye-xian, MA Yu-qian, LI Hong-hao, CHEN Gang. Fabrication of NiFe2O4/C hollow spheres constructed by mesoporous nanospheres for high-performance lithium-ion batteries [J]. Chemical Engineering Journal, 2016, 303: 501-510.
[3] MAITI D, SAHA A, DEVI P S. Surface modified multifunctional ZnFe2O4 nanoparticles for hydrophobic and hydrophilic anti-cancer drug molecule loading [J]. Physical Chemistry Chemical Physics, 2016, 18(3): 1439-1450.
[4] HASHEMINIASARI M, MASOUDPANAH S M, MIRKAZEMI S M, BAYAT F. Structural and magnetic properties of ZnFe2-xInxO4 nanoparticles synthesized by solution combustion method [J]. Journal of Magnetism & Magnetic Materials, 2017, 442: 468-473.
[5] LI Fa-shen, WANG Hai-bo, WANG Li, WANG Jian-bo. Magnetic properties of ZnFe2O4 nanoparticles produced by a low-temperature solid-state reaction method [J]. Journal of Magnetism & Magnetic Materials, 2007, 309(2): 295-299.
[6] NIYAIFAR M. Effect of preparation on structure and magnetic properties of ZnFe2O4 [J]. Journal of Magnetics, 2014, 19(2): 101-105.
[7] SINGH S, KUMAR N, JHA A, SAHNI M, BHARGAVA R, CHAWLA A, CHANDRA R, KUMAR S, CHAUBEY S. Effect of annealing temperature on the physical properties of Zn-ferrite Nanoparticles [J]. Journal of Superconductivity and Novel Magnetism, 2014, 27(3): 821-826.
[8] CAO Lei-jian, ZHOU Qing-hua, GU Li, SHEN Hong-xia, LI Qing-hua, LAN Ping, FANG Yan. Preparation and characterization of composite microspheres of nano zinc ferrite/poly (D, L-lactide-co-alanine) [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(2): 360-365.
[9] NAYAK H. Effect of catalytic activities of mixed nano ferrites of zinc and copper on decomposition kinetics of lanthanum oxalate hydrate [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(3): 767-774.
[10] WANG Yan, ZHU Hong-yu, CHEN Yan-bo, Wu Xin-ming, ZHANG Wen-zhi, LUO Chun-yan, LI Jin-hua. Design of hollow ZnFe2O4 microspheres@graphene decorated with TiO2 nanosheets as a high-performance low frequency absorber [J]. Materials Chemistry & Physics, 2017, 202: 184-189.
[11] ZHANG Na, HUANG Ying, ZONG Meng, DING Xiao, LI Su-ping, WANG Ming-yue. Synthesis of core-shell ZnFe2O4@SiO2 hollow microspheres/reduced graphene oxides for a high-performance EM wave absorber [J]. Ceramics International, 2016, 42(16): 18879- 18886.
[12] CULLITY B D. Elements of X-ray diffraction [M]. 2nd ed. Massachusetts: Addison Wesley, 1978.
[13] PHILIP J, GNANAPRAKASH G, PANNEERSELVAM G, ANTONY M P, JAYAKUMAR T, RAJ B. Effect of thermal annealing under vacuum on the crystal structure, size, and magnetic properties of ZnFe2O4 nanoparticles [J]. Journal of Applied Physics, 2007, 102(5): 054305.
[14] HAMDEH H H, HO J C, OLIVER S A , WILLEY R J, OLIVERI G, BUSCA G. Magnetic properties of partially-inverted zinc ferrite aerogel powders[J]. Journal of Applied Physics, 1997, 81(4): 1851-1857.
[15] BINI M, TONDO C, CAPSONI D, MOZZATI M C, ALBINI B, GALINETTO P. Superparamagnetic ZnFe2O4 nanoparticles: The effect of Ca and Gd doping [J]. Materials Chemistry & Physics, 2017, 204: 72-82.
[16] YADAV R S, HAVLICA J, KURITKA I, KOZAKOVA Z, PALOU M, BARTONICKOVA E, BOHAC M, FRAJKOROVA F, MASILKO J, HAJDUCHOVA M. Magnetic properties of ZnFe2O4 nanoparticles synthesized by starch-assisted sol–gel auto-combustion method [J]. Journal of Superconductivity and Novel Magnetism, 2015, 28(4): 1417-1423.
[17] SIVAGURUNATHAN P, SATHIYAMURTHY K. Effect of temperatures on structural, morphological and magnetic properties of zinc ferrite nanoparticles [J]. Canadian Chemical Transactions, 2016, 4(2): 244-254.
[18] LI Jun-qi, LIU Zhen-xing, ZHU Zhen-feng. Magnetically separable ZnFe2O4, Fe2O3/ZnFe2O4 and ZnO/ZnFe2O4 hollow nanospheres with enhanced visible photocatalytic properties [J]. Rsc Advances, 2014, 4(93): 51302-51308.
[19] ILANOVIc M, MOSHOPOULOU E G, STAMOPOULOS D, DEVLIN E, GIANNAKOPOULOS K P, KONTOS A G, ELEFTHERIADIS K, GINI M I, NIKOLIC L M. Structure and magnetic properties of Zn1-xInxFe2O4 and ZnYxFe2-xO4 nanoparticles prepared by coprecipitation [J]. Ceramics International, 2013, 39(3): 3235-3242.
ZnFe2O4空心球的制备及由顺磁到铁磁性的转变
葛毅成,王志龙,易茂中,冉丽萍
中南大学 粉末冶金国家重点实验室,长沙 410083
摘 要:采用简单浸渍法,以制备的酚醛(PF)球为模板,经500~700 °C退火,合成粒径为200~302 nm的ZnFe2O4空心球(ZFHs)。所制备的ZFHs由尺寸15~20 nm的大量小纳米粒子组装而成,在这些纳米粒子之间存在着许多介孔。经500~550 °C退火后,ZFHs呈单一的立方尖晶石结构,而较高的退火温度导致六方氧化锌和菱形α-Fe2O3二次相的形成。纳米粒子的晶粒尺寸随退火温度的升高而增大。退火温度的降低导致顺磁性向铁磁性的新转变,饱和磁化强度从2.3 A·m2/kg显著提高到13.5 A·m2/kg。研究空心球体结构的形成对Fe3+和Zn2+在尖晶石结构中再分布的影响。
关键词:ZnFe2O4空心球;纳米粒子;磁性;磁转变
(Edited by Bing YANG)
Foundation item: Project (51574293) supported by the National Natural Science Foundation of China; Project supported by the Independent Research Program of State Key Laboratory of Powder Metallurgy, China
Corresponding author: Yi-cheng GE; Tel: +86-13574105471; E-mail: hncsgyc@csu.edu.cn
DOI: 10.1016/S1003-6326(19)65057-0
Abstract: ZnFe2O4 hollow spheres (ZFHs) with sizes of 200-302 nm were synthesized by simple impregnating method using the as-prepared phenolic formaldehyde (PF) spheres as templates and subsequent annealing at 500-700 °C. The prepared ZFHs are assembled by a large number of small nanoparticles with sizes of 15-20 nm, and many mesopores exist among these nanoparticles. The samples annealed at 500-550 °C exhibit a single cubic spinel structure, while higher annealing temperature leads to the formation of hexagonal ZnO and rhombohedral α-Fe2O3 secondary phases. The size of the assembled nanoparticles increases with the increase in annealing temperature. Novel magnetic transformation from paramagnetic to ferrimagnetic is induced by the reduction of annealing temperature and the saturation magnetization significantly increases from 2.3 to 13.5 A·m2/kg. The effect of the formation of hollow sphere structure on the redistribution of Fe3+ and Zn2+ in the spinel structure was studied.


