
J. Cent. South Univ. (2018) 25: 516-525
DOI: https://doi.org/10.1007/s11771-018-3756-1

Differences in functional traits and reproductive allocations between native and invasive plants
WANG Cong-yan(王从彦), ZHOU Jia-wei(周嘉伟), LIU Jun(刘君),XIAO Hong-guang(肖鸿光), WANG Lei(王磊)
Institute of Environment and Ecology, Academy of Environmental Health and Ecological Security & School of the Environment and Safety Engineering, Jiangsu University, Zhenjiang 212013, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
Because co-occurring native and invasive plants are subjected to similar environmental selection pressures, the differences in functional traits and reproductive allocation strategies between native and invasive plants may be closely related to the success of the latter. Accordingly, this study examines differences in functional traits and reproductive allocation strategies between native and invasive plants in Eastern China. Plant height, branch number, reproductive branch number, the belowground-to-aboveground biomass ratio, and the reproductive allocation coefficient of invasive plants were all notably higher than those of native species. Additionally, the specific leaf area (SLA) values of invasive plants were remarkably lower than those of native species. Plasticity indexes of SLA, maximum branch angle, and branch number of invasive plants were each notably lower than those of native species. The reproductive allocation coefficient was positively correlated with reproductive branch number and the belowground-to-aboveground biomass ratio but exhibited negative correlations with SLA and aboveground biomass. Plant height, branch number, reproductive branch number, the belowground-to-aboveground biomass ratio, and the reproductive allocation coefficient of invasive plants may strongly influence the success of their invasions.
Key words:
Cite this article as:
WANG Cong-yan, ZHOU Jia-wei, LIU Jun, XIAO Hong-guang, WANG Lei. Differences in functional traits and reproductive allocations between native and invasive plants [J]. Journal of Central South University, 2018, 25(3): 516–525.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3756-11 Introduction
Plants inhabit multivariate environments, thus, the response of their functional traits to changing environmental factors can enable them to broaden their habitat niches and therefore employ successful ecological strategies [1–5]. As one of the most important functional traits, specific leaf area (SLA, defined as biomass investment per unit of light-capture surface area) can indicate the resource-use strategy of plants [1, 6]. Generally, high SLA values indicate high resource acquisition and use efficiency with relatively low investments in leaf construction and protective tissues [1, 2, 6].
Similarly, sexual reproduction is also an essential element of plant life histories. It strongly affects the dynamics of plant populations [7] and changes in reproductive allocation strategies (i.e., the proportion of biomass allocated to reproductive structures) are fundamental to adaptive responses to environments [4, 5, 7–10]. Increased reproduction allocations typically increase fitness [10, 11]. Thus, the growth and reproduction strategies of plants are optimized on the basis of individual fitness, ultimately increasing the fitness of each subsequent generation [7, 8].
Anthropogenic activities have triggered unprecedented global environmental changes such as biological invasions [12, 13]. Co-occurring native and invasive plants endure similar environmental selection pressures (i.e., habitat filtering); thus, differences in functional traits and reproductive allocation strategies between native and invasive plants may strongly impact the success of the latter [11, 14]. Determining the differences in functional traits and reproductive allocation strategies between native and invasive plants can illuminate the mechanisms underlying the success of plant invasions. As such, identifying which traits effectively differentiate native from invasive plants is an important goal for improving invasive weed risk assessment [4, 15].
This study examined five invasive plants (Bidens pilosa, Erigeron annuus, Conyza canadensis, Solidago canadensis, and Phytolacca americana) and five native species (Sonchus oleraceus, Hemistepta lyrata, Youngia japonica, Cirsium setosum, and Lactuca indica) because of their common occurrence throughout the study sites. Specifically, this study aims to clarify the differences in functional traits and reproductive allocation strategies between native and invasive plants and then assess the role that each factor played throughout the invasion process. We consider several hypotheses. First, SLA values of the invasive plants may be higher than those of native species because invasive plants should invest more biomass on leaf growth and total surface area rather than leaf structural tissue per unit area in order to achieve higher growth rates [16, 17]. Previous studies have shown that higher SLA is often associated with a growth advantage for invasive plants over native species [18]. Second, invasive plants may be more likely to exhibit a higher reproductive allocation coefficient than those of native species because invasive plants must consume more resources and produce more offspring through increased reproductive allocation into sexual reproduction in order to colonize a wide variety of environments [19]. Previous studies have also shown that high reproductive allocation is associated with the invasiveness of invasive plants [4, 11]. Third, SLA may be positively correlated with reproductive allocation coefficients because species with high SLA exhibit significantly higher reproductive outputs than those with lower SLA [20], in accord with allocation theory [21]. Fourth, the reproductive allocation coefficient may be positively correlated with their biomass because plants invest more biomass into reproduction when they acquire and use more resources [22]. Fifth, the plasticity indexes of invasive plants may be higher than those of native species because plants exhibit higher phenotypic plasticity in response to changes in environmental factors such that individual plants maximize their fitness across a range of environmental factors [11, 16, 23]. Previous research has also revealed positive correlations between phenotypic plasticity and the invasiveness of plant species [11, 24].
2 Materials and methods
2.1 Experimental design
From mid-May to mid-September 2014, the samples of five invasive plants, namely, B. pilosa, E. annuus, C. canadensis, S. canadensis, and P. americana, respectively, and five native plants, namely, S. oleraceus, H. lyrata, Y. japonica, C. setosum, and L. indica, respectively, were collected during their reproductive phases in Zhenjiang, China, an area characterized by a subtropical humid climate. The annual mean temperature in the area is approximately 15.9 °C, and its monthly temperature reaches a mean maximum of 28 °C in July and decreases to a mean minimum of 2.9 °C in January. The annual cumulative precipitation is approximately 1101.4 mm, and the rainy season occurs in June and July. Samples from sixteen individual plants were collected for each species at random from different sites (except for B. pilosa, of which only thirteen samples were collected). Five adult leaves from each of these individuals were selected at random in order to measure a series of leaf traits. All samples were stored in sealed bags and immediately transported back to the laboratory for measurement.
2.2 Determination of plant characteristics and plant diversity
Plant height, crown diameter, and maximum branch length were measured using a ruler. SLA was calculated as the ratio of leaf area to the leaf dry mass (cm2/g) of a given leaf, in accord with previous studies [2, 3, 25]. The maximum branch angle was measured using a protractor. The reproductive investment ratio was computed using the ratio of the number of reproductive branches to the total number of branches. Plant biomass (fresh weight) was measured using an electronic balance. The belowground-to-aboveground biomass ratio was computed by dividing the belowground biomass by the aboveground biomass of a given plant. The total reproductive investment was calculated using the biomass of reproductive organs. The reproductive allocation coefficient was computed using the ratio of reproductive organ biomass to the total biomass [26].
The plasticity index, ranging from zero (no plasticity) to one (maximum plasticity), of characteristics measured from the ten species was calculated. This index is the ratio of the absolute difference between the maximum value of one functional trait and the minimum value of the same functional trait to the maximum value of the same functional trait according to previous studies [16].
Shannon–Wiener diversity (H'), Simpson dominance (D), and Pielou’s evenness (EH) indexes were used to estimate the community structure of the survey sites (1 m×1 m quadrats). H' was determined by the equation 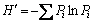 [27], where Pi is the relative abundance of a given species. Pi was calculated as Pi=ni/N, where ni is the number of individuals of a given species and N is the sum of the number of all species in the surveyed area. D was calculated as
[27], where Pi is the relative abundance of a given species. Pi was calculated as Pi=ni/N, where ni is the number of individuals of a given species and N is the sum of the number of all species in the surveyed area. D was calculated as 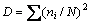 [28]. EH was calculated as EH=H'/lnS, where S is defined as the number of plant species in the surveyed area [29].
[28]. EH was calculated as EH=H'/lnS, where S is defined as the number of plant species in the surveyed area [29].
2.3 Statistical analysis
Differences among various dependent variables were assessed using an analysis of variance between groups. The statistical significance threshold for all test statistics was met when P values were equal to or less than 0.05. Correlation analysis was performed using Pearson product-moment correlation coefficient to determine the patterns among various dependent variables. All statistical analyses were performed using SPSS (version 22.0; IBM Corp., Armonk, NY, USA).
3 Results
Plant height, branch number, reproductive branch number, the belowground-to-aboveground biomass ratio, and the reproductive allocation coefficient of invasive plants were significantly higher than those of native species (Table 1, P< 0.05). However, the SLA estimates of invasive plants were significantly lower than those of native species (Table 1, P<0.05). The differences in other indexes of functional traits between native and invasive plants were not significant (Table 1, P>0.05).
The D values of the community structure of the sites in which invasive plants growing were significantly lower than those of native species (Table 1, P < 0.05). The differences in H' and EH between native and invasive plants were not significant (Table 1, P>0.05).
Plasticity indexes of SLA, maximum branch angle, branch number of invasive plants were notably lower than those of native species (Table 2, P<0.05). The differences in plasticity indexes of other functional trait indexes between native and invasive plants were not significant (Table 2, P>0.05).
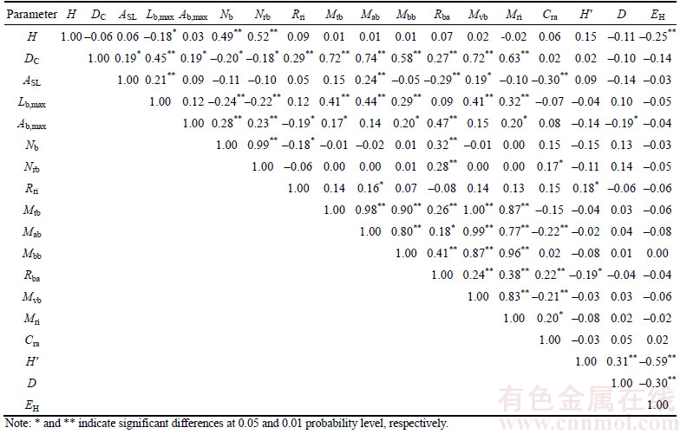
Correlation patterns among functional traits and reproductive allocation strategies of the ten species and plant diversity of the community structure of the sites were assessed via a correlation analysis (Table 3). Many significant correlations were revealed. In particular, height was positively correlated with branch number, and reproductive branch number (Table 3, P<0.0001) but exhibited negative correlations with the maximum branch length and EH values of the community structure of the sites (Table 3, P<0.05). Crown diameter was positively correlated with SLA, maximum branch length, maximum branch angle, the reproductive investment ratio, total biomass, aboveground biomass, belowground biomass, the belowground- to-aboveground biomass ratio, vegetative biomass, and total reproductive investment (Table 3, P<0.05) but exhibited negative correlations with branch number and reproductive branch number (Table 3, P<0.05). SLA was positively correlated with maximum branch length, aboveground biomass, and vegetative biomass (Table 3, P<0.05) but negatively correlated with the belowground-to- aboveground biomass ratio, and the reproductive allocation coefficient (Table 3, P<0.001). Maximum branch length was positively correlated with total biomass, aboveground biomass, belowground biomass, vegetative biomass, and total reproductive investment (Table 3, P<0.001) but negatively correlated with branch number and reproductive branch number (Table 3, P<0.01). Maximum branch angle was positively correlated with branch number, reproductive branch number, total biomass, belowground biomass, the belowground-to- aboveground biomass ratio, and total reproductive investment (Table 3, P<0.05) but negatively correlated with the reproductive investment ratio and the D value of the community structure of the sites (Table 3, P<0.05). Branch number was positively correlated with reproductive branch number and the belowground-to-aboveground biomass ratio (Table 3, P<0.0001) but negatively correlated with the reproductive investment ratio (Table 3, P<0.05). Reproductive branch number was positively correlated with the belowground-to- aboveground biomass ratio and the reproductive allocation coefficient (Table 3, P<0.05). The reproductive investment ratio was positively correlated with aboveground biomass and the H' value of the community structure of the sites (Table 3, P<0.05). There were significantly positive correlations among total biomass, aboveground biomass, belowground biomass, the belowground- to-aboveground biomass ratio, vegetative biomass, and total reproductive investment (Table 3, P< 0.05). Aboveground biomass was negatively correlated with the reproductive coefficient (Table 3, P<0.01).
Table 1 Differences in functional traits and reproductive allocation strategies between native and invasive plants, plant diversity of the community structure of the sites, and soil pH values
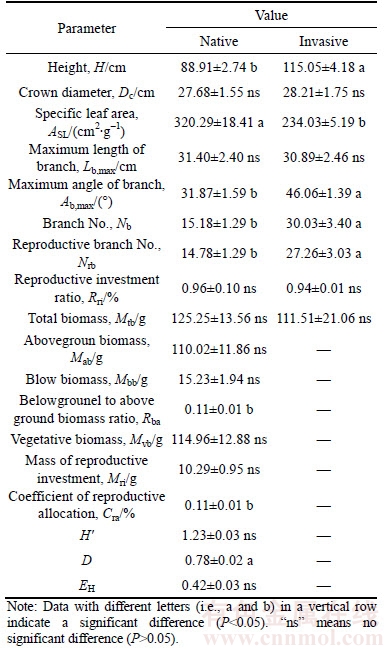
Table 2 Differences in plasticity indexes of functional traits and reproductive allocation strategies between native and invasive plants (Abbreviations have the same meanings as described in Table 1)
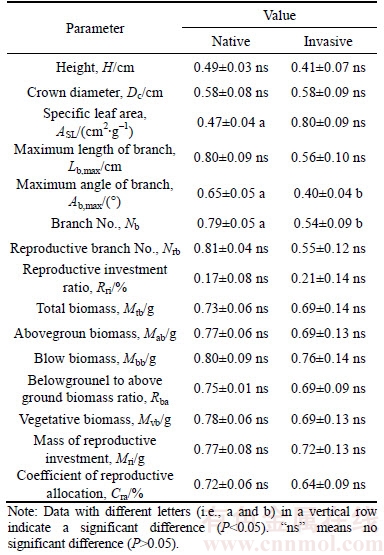
Table 3 Relationship among functional traits and reproductive allocation strategies of ten species, plant diversity of community structure of sites, and soil pH values
4 Discussion
Plant height plays an important role in the competitive ability of most plants [30]. This study demonstrated that invasive plants were notably taller than native species. Taller invasive plants may exhibit greater competitive ability in acquiring resources, particularly light, which may be the most crucial ecological factor affecting plant development, growth and survival [31].
As one of the key parameters that characterizes invasiveness and fitness [32], the total biomass of invasive plants is perhaps obviously expected to be higher than that of native species. However, invasive plants surveyed in this work exhibited biomasses that were similar to those of native species. This may be caused by any one of the factors that may have resulted in invasive plants not accumulating more biomass in their growth processes than native species. This work shows that invasive plants exhibit higher belowground-to- aboveground biomass ratios than native species, which is consistent with previous study [33]. The belowground-to-aboveground biomass ratio is commonly used to describe the biomass allocation between belowground and aboveground plant organs. There is a trade-off between belowground and aboveground resource capture efficiency, and higher belowground-to-aboveground biomass ratios can enable invasive plants to obtain a competitive advantage in nutrient and water uptake [33].
Previous investigators have shown that invasive plants invest more biomass on leaf growth rather than leaf structures per unit area and thus achieve a higher growth rate [11, 16, 18]. Moreover, higher SLA often highly correlates with a growth advantage for invasive plants over native species [18]. Thus, it is expected that SLA values of invasive plants may be higher than those of native species. In contrast with the first hypothesis, however, the results of this study show that the SLA of invasive plants is evidently lower than native species. This indicates that invasive plants do not demonstrate higher or even lower resource capture ability than native species [2], suggesting that these traits may not necessarily contribute to the success of biological invasions. Previous studies have also shown that physiological traits are not necessarily responsible for increasing the invasiveness of species [34]. Although plants can improve their relative growth rates by increasing SLA, which increases leaf transpiration rates, this thereby increases sensitivity to water stress [35]. In particular, plants may reduce SLA under water stress [35] leading to greater water use efficiency [35, 36].
In this work, invasive plants were notably taller than native species. Increased plant height may have served as a strategy to improve competition for light, but it can also impose costs of structural support and water transport [37, 38]. Thus, plants also achieve a high relative growth rate by decreasing SLA, especially under water stress [35]. At the same time, invasive plants exhibit higher water use efficiency than native species [39, 40]. Moreover, higher SLA may also require a relatively higher investment in Rubisco [41] and low resilience to unfavorable environmental conditions [1, 6]. Lower SLA values generally are associated with longer leaf life spans and thereby a greater return time on investment [18, 42], which can increase photosynthesis and net carbon gain [42, 43] for invasive plants. Yet, there is a growing body of evidence suggesting that the difference in SLA between native and invasive plants is inconsistent, i.e., invasive plants may display higher [16, 17, 44], similar [3, 33, 45], or even lower [2, 46, 47] SLA values compared with those of native species. Moreover, the relationship between relative growth rate and SLA requires special attention or even re-examination. Thus, empirical studies have presented conflicting results, including observed relationships between relative growth rate and SLA of plants that were positive [1, 48], negative [49], or variable amongst habitats [35] and growth periods [50], or not at all [46]. This suggests that there is species specificity in the relationship between relative growth rates and SLA.
As an integral component of successful life history strategies, relatively high reproductive allocation can enable plants to capture more resources and produce more offspring, thereby rapidly colonizing a wide variety of habitats [19]. Thus, invasive plants must exhibit higher reproductive allocation relative to native species in order to successfully invade ecosystems by producing high numbers of high fitness offspring. Previous studies have shown a positive relationship between reproductive capacity and invasiveness [11], and advantages related to reproductive traits are an important element of successful biological invasions [51].
The relatively high branch number, reproductive branch number, and reproductive allocation coefficient of invasive plants over those of native species are consistent with the second hypothesis. This may be a product of greater reproduction allocation enabling invasive plants to achieve higher fitness [10, 11]. Sexual reproduction, which is crucial in connecting isolated populations and colonizing new habitats via seed recruitment and dispersal, contributes much more significantly to the rapid range expansion of plants [52]. Thus, the adaptive strategy of high reproductive allocation may boost the expansion of populations via seed recruitment and dispersal, especially in competitive environments [9].
Allocation theory assumes that individuals must allocate limited resource among several competing functions such as growth, storage and reproduction [4, 5, 21]. Thus, high SLA might be associated with high reproductive output [20]. Contradicting the third hypothesis, this study revealed a negative correlation between SLA and the reproductive allocation coefficient. This suggests that there is a negative relationship between resource acquisition and reproductive allocation.
Plants with higher biomass, which can therefore allocate more biomass into reproduction, thus exhibit a higher reproductive allocation coefficient [22]. Significant positive correlations between reproductive allocation and plant size have also been revealed by previous study [4, 22]. In contrast with the fourth hypothesis, this study shows that the reproductive allocation coefficient is negatively correlated with aboveground biomass and vegetative biomass but not total biomass. There may be a negative relationship between plant biomass and reproductive allocation in this work as well as in previous studies [53]. This may be a consequence of plants investing more biomass into vegetative growth when they reach a certain size in order to support their large biomass, i.e., reproductive allocation decreases with the size of individual plants [22].
Because any functional traits that contribute to the fitness advantages of a plant in its habitat will be under selection and may thus evolve adaptively, phenotypic plasticity is a potential target for selection [1, 54]. Thus, the phenotypic plasticity of plants for any functional traits related to their performance and fitness may play an immense role in their success and survival [1, 11, 54]. This study demonstrated that all functional traits assessed for each of the ten species displayed phenotypic plasticity. Those functional traits that displayed phenotypic plasticity are likely to be involved in local adaptation of plants to their multivariate environments. Phenotypic plasticity of functional traits in response to environment changes may be part of successful ecological strategies that allow plants to live in their competitive ecosystems by broadening their habitat niches. Thus, the phenotypic plasticity of functional traits of invasive plants may be higher than those of native species. Accordingly, phenotypic plasticity is likely to play an important role in the success of biological invasions [24]. However, the result of this study, i.e., that SLA, maximum branch angle, and branch number of invasive plants were unexpectedly lower than those of native species, which is not consistent with the fifth hypothesis. The reason for the lower phenotypic plasticity of SLA, maximum branch angle, and branch number of invasive plants may be ascribed to a fitness cost for plastic plant species under unfavorable environments. In particular, the lower plasticity of functional traits can effectively compensate the negative effects of the adverse environments on plant growth [55, 56]. Consequently, the lower phenotypic plasticity of some traits for invasive plants may be one of adaptive strategies which can enhance their competitive ability and then facilitate the further invasion [2, 3, 56]. Phenotypic plasticity is important for plants as it allows them to grow in a wide range of environments; however, a large number of studies have shown that the relationships between phenotypic plasticity and plant invasion are inconsistent. Some studies have shown that invasive plants possess higher plasticity than native species, and higher phenotypic plasticity has been thought to facilitate invasion [11, 16]. Other studies have even shown that invasive plants exhibit similar [33, 57] or even lower [2, 3, 44] levels of phenotypic plasticity in comparison to native species because the lower plasticity of functional traits can compensate for the negative effects of adverse environments and thus facilitate invasion [44, 58]. As such, phenotypic plasticity may play a pivotal role in the successful invasion of some invasive plants, but perhaps not all invasive species.
5 Conclusions
Overall, successfully ecological strategies of plants achieve an optimal trade-off between growth and reproduction in order to gain more resources while optimizing fitness across changing environments. In this work, plant height, branch number, reproductive branch number, the belowground-to-aboveground biomass ratio, and the reproductive allocation coefficient of invasive plants play an important role in the successful invasions of the invasive plants examined.
References
[1] POORTER H, NIINEMETS U, POORTER L, WRIGHT I J, VILLAR R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis [J]. New Phytologist, 2009, 182(3): 565–588. DOI: 10.1111/j.1469-8137.2009. 02830.x.
[2] WANG C Y, ZHOU J W, LIU J, JIANG K. Differences in functional traits between invasive and native Amaranthus species under different forms of N deposition [J]. Science of Nature 2017, 104(7, 8): 59. DOI: 10.1007/s00114-017- 1482-4.
[3] WANG C Y, LIU J, ZHOU J W, XIAO H G. Differences in leaf functional traits between exotic and native composite plant species [J]. Journal of Central South University, 2017, 24(10): 2468–2474. DOI: 10.1007/s11771-017-3658-7.
[4] WANG C Y, ZHOU J W, LIU J, XIAO H G, WANG L. Functional traits and reproductive allocation strategy of Conyza canadensis as they vary by invasion degree along a latitude gradient [J]. Polish Journal of Environmental Studies 2017, 26(3): 1289–1297. DOI: 10.15244/pjoes/66175.
[5] WANG C Y, ZHOU J W, LIU J, WANG L, XIAO H G. Reproductive allocation strategy of two herbaceous invasive plants across different cover classes [J]. Polish Journal of Environmental Studies, 2017, 26(1): 355–364. DOI: 10.15244/pjoes/64240.
[6] PIETSCH K A, OGLE K, CORNELISSEN J H C, CORNWELL W K, B NISCH G, CRAINE J M, JACKSON B G, KATTGE J, PELTZER D A, PENUELAS J, REICH P B, WARDLE D A, WEEDON J T, WRIGHT I J, ZANNE A E, WIRTH C. Global relationship of wood and leaf litter decomposability: The role of functional traits within and across plant organs [J]. Global Ecology and Biogeography, 2014, 23(9): 1046–1057. DOI: 10.1111/geb.12172.
NISCH G, CRAINE J M, JACKSON B G, KATTGE J, PELTZER D A, PENUELAS J, REICH P B, WARDLE D A, WEEDON J T, WRIGHT I J, ZANNE A E, WIRTH C. Global relationship of wood and leaf litter decomposability: The role of functional traits within and across plant organs [J]. Global Ecology and Biogeography, 2014, 23(9): 1046–1057. DOI: 10.1111/geb.12172.
[7] WANG X Z, TAUB D R, JABLONSKI L M. Reproductive allocation in plants as affected by elevated carbon dioxide and other environmental changes: A synthesis using meta-analysis and graphical vector analysis [J]. Oecologia, 2015, 177(4): 1075–1087. DOI: 10.1007/s00442-014- 3191-4.
[8] TIAN D S, PAN Q M, SIMMONS M, CHAOLU H, DU B H, BAI Y F, WANG H, HAN X G. Hierarchical reproductive allocation and allometry within a perennial bunchgrass after 11 years of nutrient addition [J]. PLoS ONE, 2012, 7(9): e42833. DOI: 10.1371/journal.pone.0042833.
[9] BONSER S P. High reproductive efficiency as an adaptive strategy in competitive environments [J]. Functional Ecology, 2013, 27(4): 876–885. DOI: 10.1111/1365-2435.12064.
[10] WANG P, WEINER J, CAHILL J R J F, ZHOU D W, BIAN H F, SONG Y T, SHENG L X. Shoot competition, root competition and reproductive allocation in Chenopodium acuminatum [J]. Journal of Ecology, 2014, 102(6): 1688–1696. DOI: 10.1111/1365-2745.12313.
[11] VAN KLEUNEN M, WEBER E, FISCHER M. A meta-analysis of trait differences between invasive and non-invasive plant species [J]. Ecology Letters, 2010, 13(2): 235–245. DOI: 10.1111/j.1461-0248.2009.01418.x.
[12] GUREVITCH J, FOX G A, WARDLE G M, INDERJIT S, TAUB D. Emergent insights from the synthesis of conceptual frameworks for biological invasions [J]. Ecology Letters, 2011, 14(4): 407–418. DOI: 10.1111/j.1461- 0248.2011.01594.x
[13] POWELL K I, CHASE J M, KNIGHT T M. Invasive plants have scale-dependent effects on diversity by altering species-area relationships [J]. Science, 2013, 339(6117): 316–318. DOI: 10.1126/science.1226817.
[14] GAO X M, ZHAO Y J, YANG X J, SUN S C. Linking trait differences to community dynamics: Evidence from Eupatorium adenophorum and co-occurring native species during a three-year succession [J]. PLoS ONE, 2013, 8(1): e50247. DOI: 10.1371/journal.pone.0050247.
[15] GALLAGHER R V, RANDALL R P, LEISHMAN M R. Trait differences between naturalized and invasive plant species independent of residence time and phylogeny [J]. Conservation Biology, 2015, 29(2): 360–369. DOI: 10.1111/cobi.12399.
[16] LAMARQUE L J, PORT A J, EYMERIC C, LASNIER J B, LORTIE C J, DELZON S. A test for pre-adapted phenotypic plasticity in the invasive tree Acer negundo L. [J]. PLoS ONE, 2013, 8(9): e74239. DOI: 10.1371/journal.pone. 0074239.
A J, EYMERIC C, LASNIER J B, LORTIE C J, DELZON S. A test for pre-adapted phenotypic plasticity in the invasive tree Acer negundo L. [J]. PLoS ONE, 2013, 8(9): e74239. DOI: 10.1371/journal.pone. 0074239.
[17] TE BEEST M, ESLER K J, RICHARDSON D M. Linking functional traits to impacts of invasive plant species: A case study [J]. Plant Ecology, 2015, 216(2): 293–305. DOI: 10.1007/s11258-014-0437-5.
[18] LEISHMAN M R, HASLEHURST T, ARES A, BARUCH Z. Leaf trait relationships of native and invasive plants: community- and global-scale comparisons [J]. New Phytologist, 2007, 176(3): 635–643. DOI: 10.1111/j.1469- 8137.2007.02189.x.
[19]  V, PRACH K. Reproductive characteristics of neophytes in the Czech Republic: Traits of invasive and non-invasive species [J]. Preslia, 2010, 82(4): 365–390.
V, PRACH K. Reproductive characteristics of neophytes in the Czech Republic: Traits of invasive and non-invasive species [J]. Preslia, 2010, 82(4): 365–390.
[20] FENESI A, DYER A R, GER D J, S
D J, S NDOR D, RUPRECHT E. Can transgenerational plasticity contribute to the invasion success of annual plant species? [J]. Oecologia, 2014, 176(1): 95–106. DOI: 10.1007/s00442-014-2994-7.
NDOR D, RUPRECHT E. Can transgenerational plasticity contribute to the invasion success of annual plant species? [J]. Oecologia, 2014, 176(1): 95–106. DOI: 10.1007/s00442-014-2994-7.
[21] BAZZAZ F A. Allocation of resources in plants: State of the science and critical questions [M]. San Diego: Academic, Press, 1997: 1–37.
[22] WEINER J, ROSENMEIER I, MASSONI E S, VERA J N, PLAZA E H, SEBASTIA M T. Is reproductive allocation in Senecio vulgaris plastic [J]. Botany, 2009, 87(5): 475–481. DOI: 10.1139/B09-012.
[23] GRIFFITH A B, ANDONIAN K, WEISS C P, LOIK M E. Variation in phenotypic plasticity for native and invasive populations of Bromus tectorum [J]. Biological Invasions, 2014, 16(12): 2627–2638. DOI: 10.1007/s10530-014- 0692-3.
[24] HULME P. Phenotypic plasticity and plant invasions: is it all Jack? [J]. Functional Ecology, 2008, 22(1): 3–7. DOI: 10.1111/j.1365-2435.2007.01369.x.
[25] WANG C Y, ZHOU J W, JIANG K, LIU J. Differences in leaf functional traits and allelopathic effects on seed germination and growth of Lactuca sativa between red and green leaves of Rhus typhina [J]. South African Journal of Botany 2017, 111: 17–22. DOI: 10.1016/j.sajb.2017.03.019.
[26] SZYMURA M, SZYMURA T H. Growth, phenology, and biomass allocation of alien Solidago species in central Europe [J]. Plant Species Biology, 2015, 30(4): 245–256. DOI: 10.1111/1442-1984.12059.
[27] SHANNON C E, WEAVER W. The mathematical theory of communication [M]. Urbana, Illinois, University of Illinois Press, 1949: 1–117.
[28] SIMPSON E H. Measurement of diversity [J]. Nature, 1949, 163: 688.
[29] PIELOU E C. The measurement of diversity in different types of biological collections [J]. Journal of Theoretical Biology, 1966, 13: 131–144. DOI: 10.1016/0022- 5193(66)90013-0.
[30] THOMSON F J, MOLES A T, AULD T D, KINGSFORD R T. Seed dispersal distance is more strongly correlated with plant height than with seed mass [J]. Journal of Ecology, 2011, 99(6): 1299–1307. DOI: 10.1111/j.1365-2745.2011. 01867.x.
[31] MENG F Q, CAO R, YANG D M, NIKLAS K J, SUN S C. Trade-offs between light interception and leaf water shedding: A comparison of shade- and sun-adapted species in a subtropical rainforest [J]. Oecologia, 2014, 174(1): 13–22. DOI: 10.1007/s00442-013-2746-0.
[32] HWANG B C, LAUENROTH W K. Effect of nitrogen, water and neighbor density on the growth of Hesperis matronalis and two native perennials [J]. Biological Invasions, 2008, 10(5): 771–779. DOI: 10.1007/s10530-007-9171-4.
[33] FENG Y L, WANG J F, SANG W G. Biomass allocation, morphology and photosynthesis of invasive and noninvasive exotic species grown at four irradiance levels [J]. Acta Oecologica, 2007, 31(1): 40–47. DOI: 10.1016/j.actao. 2006.03.009.
[34] LAMARQUE L J, LORTIE C J, PORT A J, DELZON S. Genetic differentiation and phenotypic plasticity in life-history traits between native and introduced populations of invasive maple trees [J]. Biological Invasions, 2015, 17(4): 1109–1122. DOI: 10.1007/s10530-014-0781-3.
A J, DELZON S. Genetic differentiation and phenotypic plasticity in life-history traits between native and introduced populations of invasive maple trees [J]. Biological Invasions, 2015, 17(4): 1109–1122. DOI: 10.1007/s10530-014-0781-3.
[35] LEBEL P, BRADLEY R L, THIFFAULT N. The relative importance of nitrogen vs. moisture stress may drive intraspecific variations in the SLA-RGR relationship: The case of Picea mariana seedlings [J]. American Journal of Plant Sciences, 2013, 4(6): 1278–1284. DOI: 10.4236/ ajps.2013.46158.
[36] GOUVEIA A C, FREITAS H. Modulation of leaf attributes and water use efficiency in Quercus suber along a rainfall gradient [J]. Trees, 2009, 23(2): 267–275. DOI: 10.1007/ s00468-008-0274-z.
[37] HAMILTON M A, MURRAY B R, CADOTTE M W, HOSE G C, BAKER A C, HARRIS C J, LICARI D. Life-history correlates of plant invasiveness at regional and continental scales [J]. Ecology Letters, 2005, 8(8): 1066–1074. DOI: 10.1111/j.1461-0248.2005.00809.x
[38] ISHII H, ASANO S. The role of crown architecture, leaf phenology and photosynthetic activity in promoting complementary use of light among coexisting species in temperate forests [J]. Ecological Research, 2010, 25(4): 715–722. DOI: 10.1007/s11284-009-0668-4.
[39] JIANG L F, LUO Y Q, CHEN J K, LI B. Ecophysiological characteristics of invasive Spartina alterniflora and native species in salt marshes of Yangtze River estuary, China [J]. Estuarine, Coastal and Shelf Science, 2009, 81(1): 74–82. DOI: 10.1016/j.ecss.2008.09.018.
[40] MATZEK V. Superior performance and nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment [J]. Biological Invasions, 2011, 13(12): 3005–3014. DOI: 10.1007/s10530-011-9985-y.
[41] POORTER H, LAMBERS H, EVANS J R. Trait correlation networks: A whole-plant perspective on the recently criticized leaf economic spectrum [J]. New Phytologist, 2014, 201(2): 378–382. DOI: 10.1111/nph.12547.
[42] FENG Y L, AUGE H, EBELING S K. Invasive Buddleja davidii allocates more nitrogen to its photosynthetic machinery than five native woody species [J]. Oecologia, 2007, 153(3): 501–510. DOI: 10.1007/s00442-007-0759-2.
[43] CIANCIARUSO M V, SILVA I A, MANICA L T, SOUZA J P. Leaf habit does not predict leaf functional traits in cerrado woody species [J]. Basic and Applied Ecology, 2013, 14(5): 404–412. DOI: 10.1016/j.baae.2013.05.002.
[44] QUAN G M, MAO D J, ZHANG J E, XIE J F, XU H Q, AN M. Response of invasive Chromolaena odorata and two coexisting weeds to contrasting irradiance and nitrogen [J]. Photosynthetica, 2015, 53(3): 419–429. DOI: 10.1007/ s11099-015-0137-y.
[45] ORDONEZ A. Global meta-analysis of trait consistency of non-native plants between their native and introduced areas [J]. Global Ecology and Biogeography, 2014, 23(3): 264–273. DOI: 10.1111/geb.12123.
[46] QING H, YAO Y H, XIAO Y, HU F Q, SUN Y X, ZHOU C F, AN S Q. Invasive and native tall forms of Spartina alterniflora respond differently to nitrogen availability [J]. Acta Oecologica, 2011, 37(1): 23–30. DOI: 10.1016/j.actao. 2010.11.002.
[47] GENG X Y, JIANG S, LI B, PAN X Y. Do higher resource capture ability and utilization efficiency facilitate the successful invasion of exotic plant? A case study of Alternanthera philoxeroides [J]. American Journal of Plant Sciences, 2013, 4(9): 1839–1845. DOI: 10.4236/ajps. 2013.49226.
[48] TIAN Y H, LEI Y B, ZHENG Y L, CAI Z Q. Synergistic effect of colonization with arbuscular mycorrhizal fungi improves growth and drought tolerance of Plukenetia volubilis seedlings [J]. Acta Physiologiae Plantarum, 2013, 35(3): 687–696. DOI: 10.1007/s11738-012-1109-5.
[49] POSSEN B J H M, ANTTONEN M J, OKSANEN E, ROUSI M, HEINONEN J, KOSTIAINEN K, KONTUNEN- SOPPELA S, HEISKANEN J, VAPAAVUORI E M. Variation in 13 leaf morphological and physiological traits within a silver birch (Betula pendula) stand and their relation to growth [J]. Canadian Journal of Forest Research, 2014, 44(6): 657–665. DOI: 10.1139/cjfr-2013-0493.
[50] IIDA Y, KOHYAMA T S, SWENSON N G, SU S H, CHEN C T, CHIANG J M, SUN I F. Linking functional traits and demographic rates in a subtropical tree community: The importance of size dependency [J]. Journal of Ecology, 2014, 102(3): 641–650. DOI: 10.1111/1365-2745.12221.
[51] WALCK J L, BASKIN J M, BASKIN C C. Why is Solidago shortii narrowly endemic and S. altissmima geographically widespread? A comprehensive comparative study of biological trait [J]. Journal of Biogeography, 2001, 28(10): 1221–1237. DOI: 10.1046/j.1365-2699.2001.00620.x.
[52] LIU H Y, LIN Z S, QI X Z, ZHANG M Y, YANG H. The relative importance of sexual and asexual reproduction in the spread of Spartina alterniflora using a spatially explicit individual-based model [J]. Ecological Research, 2014, 29(5): 905–915. DOI: 10.1007/s11284-014-1181-y.
[53] WANG T H, ZHOU D W, WANG P, ZHANG H X. Size-dependent reproductive effort in Amaranthus retroflexus: the influence of planting density and sowing date [J]. Canadian Journal of Botany, 2006, 84(3): 485–492. DOI: 10.1139/B06-011.
[54] MCINTYRE P J, STRAUSS S Y. Phenotypic and transgenerational plasticity promote local adaptation to sun and shade environments [J]. Ecology and Evolution, 2014, 28(2): 229–246. DOI: 10.1007/s10682-013-9670-y.
[55] QUAN G M, MAO D J, ZHANG J E, XIE J F, XU H Q, AN M. Response of invasive Chromolaena odorata and two coexisting weeds to contrasting irradiance and nitrogen [J]. Photosynthetica, 2015, 53(3): 419–429. DOI: 10.1007/ s11099-015-0137-y.
[56] PALACIO-L AEZ K, GIANOLI E. Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis [J]. Oikos, 2011, 120(9): 1393–1401. DOI: 10.1111/j.1600-0706.2010. 19114.x.
AEZ K, GIANOLI E. Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis [J]. Oikos, 2011, 120(9): 1393–1401. DOI: 10.1111/j.1600-0706.2010. 19114.x.
[57] MATZEK V. Trait values, not trait plasticity, best explain invasive species performance in a changing environment [J]. PLoS ONE, 2012, 7(10): e48821. DOI: 10.1371/journal. pone.0048821.
[58] MARON J L, ELMENDORF S C,  M. Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum [J]. Evolution, 2007, 61(8): 1912–1924. DOI: 10.1111/j.1558-5646.2007. 00153.x.
M. Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum [J]. Evolution, 2007, 61(8): 1912–1924. DOI: 10.1111/j.1558-5646.2007. 00153.x.
(Edited by FANG Jing-hua)
中文导读
入侵植物与本地植物的功能性状和繁殖分配之差异
摘要:由于入侵植物和本地植物面临类似的生境选择压力,因此入侵植物与本地植物的功能性状和繁殖分配的差异与入侵植物的成功入侵密切相关。本文研究了华东地区入侵植物与本地植物的功能性状和繁殖分配的差异。研究结果表明:入侵植物的株高、分枝数、繁殖枝数、地下生物量/地上生物量比和繁殖分配系数显著大于本地植物的, 但是入侵植物的比叶面积显著低于本地植物的。此外,入侵植物的比叶面积、最大分枝角度和分枝数的表型可塑性指数显著低于本地植物的。繁殖分配系数与繁殖枝数和地下生物量/地上生物量比呈显著正相关,但与比叶面积和地上生物量呈显著负相关。因此,入侵植物的株高、分枝数、繁殖枝数、地下生物量/地上生物量比和繁殖分配系数在其成功入侵进程中可能起重要作用。
关键词:功能性状;比叶面积;繁殖分配策略;表型可塑性;入侵植物
Foundation item: Project(31300343) supported by the National Natural Science Foundation of China; Project supported by Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment, China; Project(12JDG086) supported by Research Foundation for Advanced Talents of Jiangsu University, China
Received date: 2016-06-16; Accepted date: 2016-09-28
Corresponding author: WANG Cong-yan, PhD, Associate Professor; Tel: +86–511–88790955; E-mail: liuyuexue623@163.com; ORCID: 0000-0002-6132-3319
Abstract: Because co-occurring native and invasive plants are subjected to similar environmental selection pressures, the differences in functional traits and reproductive allocation strategies between native and invasive plants may be closely related to the success of the latter. Accordingly, this study examines differences in functional traits and reproductive allocation strategies between native and invasive plants in Eastern China. Plant height, branch number, reproductive branch number, the belowground-to-aboveground biomass ratio, and the reproductive allocation coefficient of invasive plants were all notably higher than those of native species. Additionally, the specific leaf area (SLA) values of invasive plants were remarkably lower than those of native species. Plasticity indexes of SLA, maximum branch angle, and branch number of invasive plants were each notably lower than those of native species. The reproductive allocation coefficient was positively correlated with reproductive branch number and the belowground-to-aboveground biomass ratio but exhibited negative correlations with SLA and aboveground biomass. Plant height, branch number, reproductive branch number, the belowground-to-aboveground biomass ratio, and the reproductive allocation coefficient of invasive plants may strongly influence the success of their invasions.


