Trans. Nonferrous Met. Soc. China 24(2014) 1928-1936
Kinetics of SiO2 leaching from Al2O3 extracted slag of fly ash with sodium hydroxide solution
Ruo-chao WANG, Yu-chun ZHAI, Zhi-qiang NING, Pei-hua MA
School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 19 December 2012; accepted 6 June 2013
Abstract:
Kinetics of SiO2 leaching from Al2O3 extracted slag of fly ash with sodium hydroxide solution was studied. The effect of leaching temperature, mass ratio of NaOH to SiO2 and stirring speed on SiO2 leaching rate was investigated. The results show that increasing leaching temperature, mass ratio of NaOH to SiO2 and stirring speed increases SiO2 leaching rate. The SiO2 leaching rate is 95.66% under the optimized conditions. There are two stages for the SiO2 leaching process, and the leaching reaction is very rapid in the first stage but quite slow in the second stage. The whole leaching process follows the shrinking core model, and the outer diffusion of no product layer is the rate-controlling step. The activation energies of the first and second stages are calculated to be 8.492 kJ/mol and 8.668 kJ/mol, respectively. The kinetic equations of the first and the second stages were obtained, respectively.
Key words:
fly ash; leaching; kinetics; SiO2;
1 Introduction
Fly ash is a fine ash separated from the flue gas of coal-fired power plant burning pulverized coal [1]. The contents of Al2O3 and SiO2 in fly ash are very high. However, fly ash is mainly used in the building industry, highway road bases and grout mixes [2,3], which cause huge waste of resources. So, it will be very necessary to improve value-added utilization of fly ash. Through recovering Al2O3 and SiO2 from fly ash, the status of lacking metal resources in China would be greatly relieved.
Many typical metallurgical methods on the comprehensive utilization of fly ash have been reported. Limestone sintering method is similar to the major method currently used in the Al2O3 plant. But due to the addition of a large amount of limestone in the sintering process, a considerable amount of calcium silicate residue can be used for cement production only [4,5]. Thus the SiO2 resource is wasted. Acid leaching method has been a hot research topic in recent years [6-8]; however, by which the recovery rate of Al2O3 is lower [9-11]. The Al2O3, which exists in Al2O3 extracted slag, could decrease the quality and yield of SiO2 product. There are also some other methods. WANG et al [12,13] studied extracting SiO2 from fly ash by alkali leaching, and then Al2O3 was extracted from desiliconized fly ash by alkali lime sintering process. The recovery rates of SiO2 and Al2O3 were 72% and 90%, respectively. From the above, it can be seen that improving the recovery rate of SiO2 is the key for the utilization of fly ash.
In the present work, a novel method for the comprehensive utilization of fly ash was established, which not only takes full advantage of Al2O3, but also makes SiO2 enriched in Al2O3 extracted slag. In this method, NH4HSO4 roasting technology was used to treat fly ash, by which Al and Si can be effectively separated. The content of SiO2 in Al2O3 extracted slag could reach 90%, which is obviously higher than that in other reports (about 68.9% or 86.72%) [8,14]. In this study, the Al2O3 extracted slag was used as raw material to recover SiO2 with NaOH solution.
In the actual production process, the reaction rate and the reaction effect are mainly depended on the kinetics factors. Nevertheless, there are no reports on kinetics of SiO2 leaching from Al2O3 extracted slag of fly ash. Therefore, it is very necessary to study the kinetics of SiO2 leaching process. The objectives are to investigate the main factors involved in the leaching process, such as leaching temperature, mass ratio of NaOH to SiO2 and stirring speed, and also to determine the kinetics of SiO2 leaching from Al2O3 extracted slag.
2 Experimental
2.1 Materials
NH4HSO4 and NaOH were in industrial grade; the reagents used for analysis and detection were in analytical grade. Deionized water was used throughout the experiments whenever needed. Representative sample of fly ash was taken from a coal-fired power plant in Inner Mongolia, China, the size fraction of which mainly ranged from 61 to 74 μm. Fly ash was mixed with NH4HSO4 at a mole ratio of Al2O3 to NH4HSO4 of 1:8. The mixture was roasted at 400 °C for 60 min in air atmosphere. The roasted clinker was leached with deionized water at 90 °C for 60 min, and then filtered. The Al2O3 extracted slag was obtained. The detailed chemical compositions of fly ash and Al2O3 extracted slag were examined by ICP, and the analytical results are shown in Table 1. Compared with fly ash, the Al2O3 content in Al2O3 extracted slag is lowered while the SiO2 content is improved. The mineral phases of fly ash and Al2O3 extracted slag were analyzed by XRD. The XRD analysis was performed using Cu Kα radiation (λ=1.5406 nm) at 40 kV and 30 mA. The XRD results are shown in Fig. 1. It can be seen that the major mineral phases of fly ash are mullite and α-SiO2. At the same time, a broad characteristic diffraction peak appears from 20° to 25°, indicating the existence of glass phase.
Table 1 Compositions of fly ash and Al2O3 extracted slag (mass fraction, %)


Fig. 1 XRD patterns of fly ash and Al2O3 extracted slag
Whereas, there is no mullite phase in Al2O3 extracted slag, which shows that the Al2O3 in mullite has been extracted, and the mineral phase of Al2O3 extracted slag is α-SiO2. The morphologies of fly ash and Al2O3 extracted slag were examined by SEM. It can be seen from Fig. 2(a) that fly ash presents a rough surface. Figure 2(b) shows that there are many small holes in the particle of Al2O3 extracted slag, which were eroded by NH4HSO4.
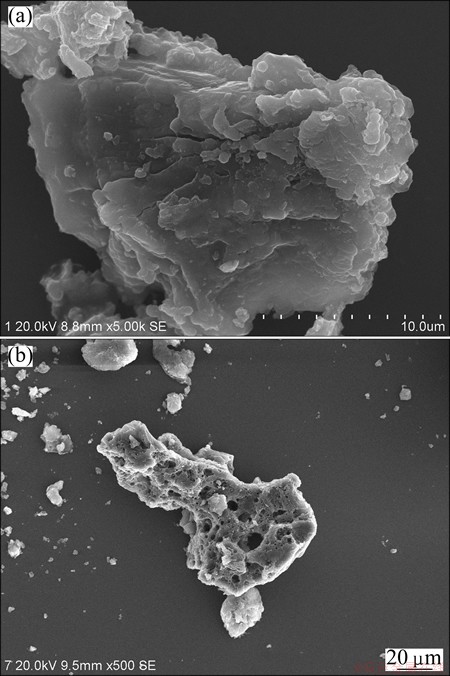
Fig. 2 SEM images of fly ash (a) and Al2O3 extracted slag (b)
2.2 Method
The leaching experiments were performed in a 1 L polytetrafluoroethylene (PTFE) beaker with a mechanical stirrer and heated in a water bath with a temperature controller. The accuracy of leaching temperature was ±0.5 °C. 50 g of Al2O3 extracted slag was used for each experiment. According to the mass ratio of NaOH to SiO2, the calculated mass of NaOH was added to the PTFE beaker containing 500 mL water, and then the NaOH solution was heated to the desired temperature. When the temperature reached and remained stable, the Al2O3 extracted slag was added under the condition of continuously stirring. After selected time intervals, approximately 10 mL of sample of the reaction mixture was taken out, and then quickly filtered. The content of SiO2 in residue was analyzed by titration, and SiO2 leaching rate was calculated as
 (1)
(1)
where α(SiO2) is the SiO2 leaching rate; m(SiO2) is the mass of SiO2 in Al2O3 extracted slag; m'(SiO2) is the mass of SiO2 in residue.
3 Results and discussion
3.1 Leaching process and controlling step for reaction rate
The main chemical reaction that occurs between Al2O3 extracted slag and NaOH is as follows:
nSiO2+2NaOH=Na2O·nSiO2+H2O (2)
Due to the existence of a small amount of Al2O3, the following reaction will take place in the leaching process:
Al2O3+2NaOH+3H2O=2NaAl(OH)4 (3)
With the increase of Al content, the reaction between NaAl(OH)4 and Na2O·nSiO2 will occur, which can be written as
Na2O·nSiO2+2NaAl(OH)4=Na2O·Al2O3·nSiO2+3H2O+2NaOH (4)
The particle of Al2O3 extracted slag is regarded as dense spherical type. During the process of SiO2 leaching from Al2O3 extracted slag, the particle surface area decreases as the reaction progresses. So, the liquid-solid leaching process can be analyzed with the shrinking core model, as shown in Fig. 3.
The reaction includes four steps as follows: 1) outer diffusion of leaching agent (NaOH solution) through the liquid boundary layer; 2) inner diffusion of leaching agent (NaOH solution) and liquid product (Na2O·nSiO2) through the residue layer; 3) chemical reaction between particle of Al2O3 extracted slag and leaching agent (NaOH solution); 4) diffusion of liquid product (Na2O·nSiO2) to liquid of leaching system.
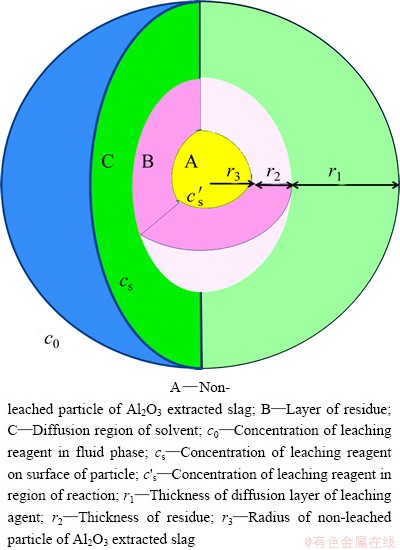
Fig. 3 Illustrative diagram of leaching process
During the process of SiO2 leaching, the main product is liquid product (Na2O·nSiO2), so the inner diffusion resistance is small. Thus the rate-controlling step is outer diffusion of no product layer or surface chemical reaction.
When the leaching process is controlled by outer diffusion of no product layer, the following expression of shrinking core model can be used to describe the kinetics [15]:
1-(1-α)2/3=k't (5)
When the leaching process is controlled by surface chemical reaction, the following expression of shrinking core model can be used to describe the kinetics [15,16]:
1-(1-α)1/3=k"t (6)
where α is the SiO2 leaching rate; k' and k" are the apparent rate constants; t is the leaching time.
3.2 Effect of leaching temperature
The influence of temperature on SiO2 leaching rate was studied in a mass ratio of NaOH to SiO2 2:1 at stirring speed of 300 r/min, and the results are shown in Fig. 4. The temperature has a noticeable influence on SiO2 leaching rate. The SiO2 leaching rate increases with the increase of leaching temperature. When the temperature increases from 25 °C to 99 °C, the leaching rate of SiO2 after 30 min improves from 34.75% to 96.08%. It seems that high temperature makes the reaction molecules move faster and strengthens the liquid-solid mass transfer. In order to achieve a better effect on SiO2 leaching reaction and avoid excessive energy consumption, leaching temperature of 90 °C is chosen. Figure 4 also shows that the leaching reaction is very rapid in 1-5 min but quite slow in 5-30 min. Such as at 90 °C, the leaching rate of SiO2 reaches 91.85% in 1-5 min, while increases by just 3.81% in 5-30 min. Thereby, the effect of leaching temperature on SiO2 leaching rate is divided into two parts: 1-5 min is the first stage; 5-30 min is the second stage [17].
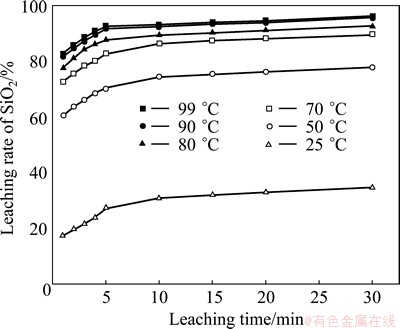
Fig. 4 Effect of leaching temperature on SiO2 leaching rate
In order to determine the kinetic parameters and rate-controlling step for SiO2 leaching from Al2O3 extracted slag, the experimental data presented in Fig. 4 were analyzed on the shrinking core model. By fitting the experimental data in Fig. 4 and Eq. (5) or Eq. (6), the results are shown in Fig. 5 and Fig.6, respectively. In Table 2, the apparent rate constants and correlation coefficient values are given for outer diffusion of no product layer and surface chemical reaction.
Table 2 shows that the apparent rate constants of the first stage are bigger than that of the second stage in the two rate-controlling steps, and there is an order of magnitude between the two stages. It also illustrates that the leaching reaction is very rapid in the first stage but quite slow in the second stage in the experimental temperature range.
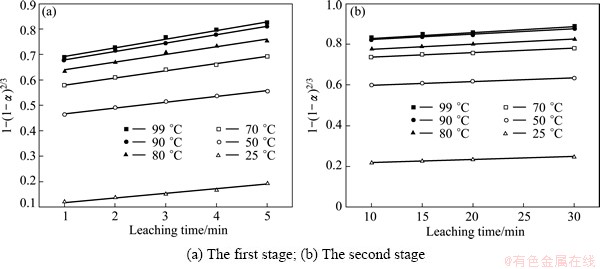
Fig. 5 Plots of 1-(1-α)2/3 vs time at different temperatures
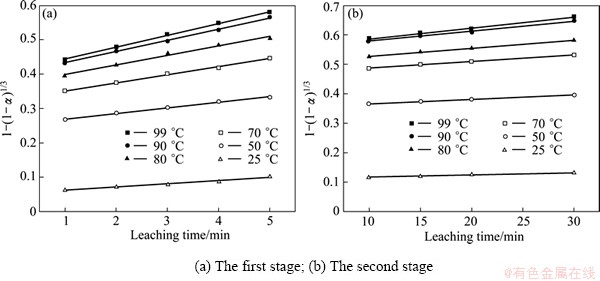
Fig. 6 Plots of 1-(1-α)1/3 vs time at different temperatures
Table 2 Apparent rate constants and correlation coefficients at different temperatures

According to Arrhenius equation, it is represented as [18]
ln k=ln A-E/(RT) (7)
where k is the reaction rate constant; A is the pre-exponential factor; E is the reaction activation energy; R is the mole gas constant; T is the thermodynamic temperature.
To calculate the activation energy, the experimental data presented in Table 2 were used to make a plot of ln k vs T-1. The Arrhenius plots of outer diffusion of no product layer and surface chemical reaction are shown in Fig. 7 and Fig. 8, respectively. And the activation energies are listed in Table 3.
Table 3 shows that when the SiO2 leaching process is controlled by outer diffusion of no product layer, the reaction activation energies of the first and second stages are 8.492 kJ/mol and 8.668 kJ/mol, respectively. Whereas, when the SiO2 leaching process is controlled by surface chemical reaction, the reaction activation energies of the first and second stages are 16.566 kJ/mol and 19.088 kJ/mol, respectively. As we all know, the activation energy of outer diffusion controlled process is typically between 4 and 12 kJ/mol [15,16], while, for a chemically controlled process, the value is usually greater than 41.8 kJ/mol [15]. Therefore, the value of activation energy indicates that the SiO2 leaching process from Al2O3 extracted slag with NaOH solution is controlled by outer diffusion of no product layer.
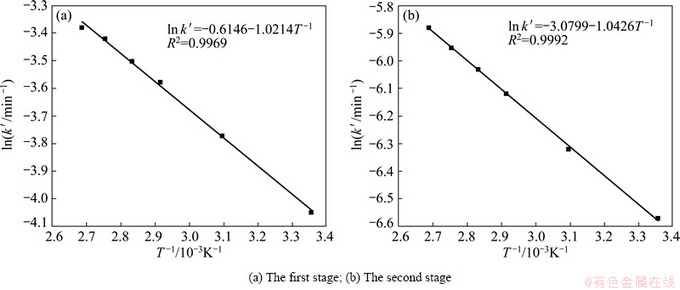
Fig. 7 Arrhenius plot of outer diffusion controlling
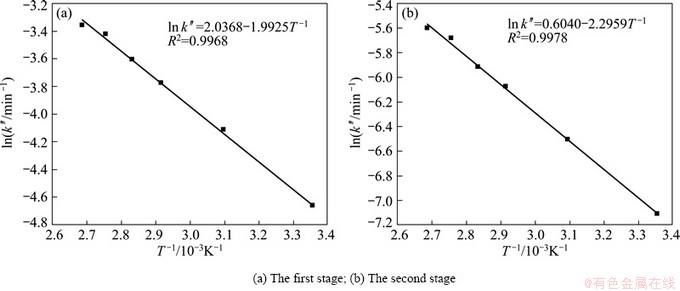
Fig. 8 Arrhenius plot of surface chemical reaction controlling
Table 3 Activation energies and kinetic equations of SiO2 leaching from Al2O3 extracted slag

3.3 Effect of mass ratio of NaOH to SiO2
The effect of the mass ratio of NaOH to SiO2 on SiO2 leaching rate was examined under the conditions of leaching temperature 90 °C and stirring speed 300 r/min, and the results are shown in Fig. 9. When the mass ratio of NaOH to SiO2 increases from 1:1 to 3:1, the SiO2 leaching rate improves with increasing the mass ratio of NaOH to SiO2. Excess NaOH can make the reaction molecules contact better and ensure sufficient reactions. However, when the mass ratio of NaOH to SiO2 is 4:1, the reaction between NaOH and Al2O3 is accelerated. When the concentration of Al in the solution reaches a certain value, NaAl(OH)4 could react with Na2O·nSiO2 to generate sodium-silicon slag, which will lead to the decrease of SiO2 leaching rate. When the mass ratio of NaOH to SiO2 is 2:1, SiO2 leaching reaction can achieve a better effect and the cost is low, so the mass ratio of NaOH to SiO2 of 2:1 is chosen. Figure 9 shows that leaching reaction is rapid in 1-5 min while slow in 5-30 min. Therefore, the effect of mass ratio of NaOH to SiO2 on SiO2 leaching rate is also divided into two parts.
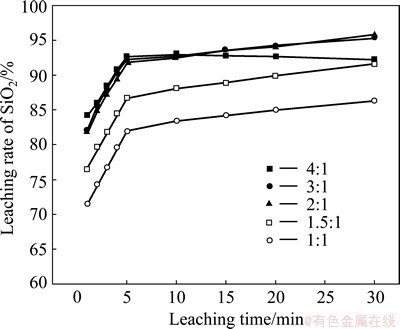
Fig. 9 Effect of mass ratio of NaOH to SiO2 on SiO2 leaching rate
The experimental data presented in Fig. 9 were brought into Eq. (5) for linear fitting, and the results are shown in Fig. 10. It can be seen that there is a good linear relationship between 1-(1-α)2/3 and time. It is found that the SiO2 leaching process is controlled by outer diffusion of no product layer at different mass ratios of NaOH to SiO2. The apparent rate constants and correlation coefficient values are shown in Table 4.
3.4 Effect of stirring speed
Experiments with various stirring speeds were carried out under typical test conditions of leaching temperature 90 °C and mass ratio of NaOH to SiO2 2:1. The results are shown in Fig. 11. It is found that the stirring speed has a remarkable influence on SiO2 leaching rate. The SiO2 leaching rate increases with the increase of stirring speed. A typical characteristic of outer diffusion is that increasing stirring speed increases the leaching rate [16]. Thus, Fig. 11 also illustrates that the rate-controlling step of SiO2 leaching process is outer diffusion. When the stirring speed is 300 r/min, SiO2 leaching reaction can achieve a good effect and the energy consumption is low, so the stirring speed of 300 r/min is chosen. Figure 11 shows that SiO2 leaching rate is rapid in 1-5 min while slow in 5-30 min. Therefore, the effect of stirring speed on SiO2 leaching rate is also divided into two parts.
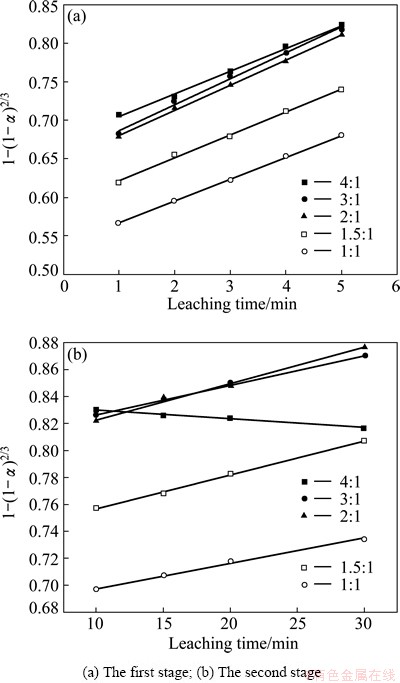
Fig. 10 Plots of 1-(1-α)2/3 vs time at different mass ratios of NaOH to SiO2
Table 4 Apparent rate constants and correlation coefficients at different mass ratios of NaOH to SiO2

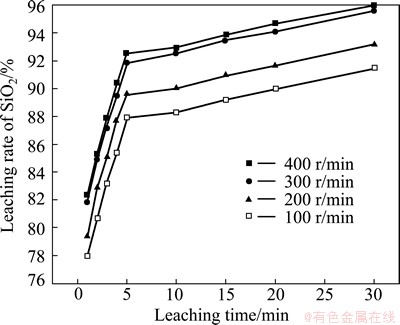
Fig. 11 Effect of stirring speed on SiO2 leaching rate
The experimental data presented in Fig. 11 were brought into Eq. (5) for linear fitting, and the results are shown in Fig. 12. It is evident that there is a good linear relationship between 1-(1-α)2/3 and time. This result manifests that the SiO2 leaching process is controlled by outer diffusion of no product layer at different stirring speeds. The apparent rate constants and correlation coefficients are shown in Table 5.

Fig. 12 Plots of 1-(1-α)2/3 vs time at different stirring speeds
Table 5 Apparent rate constants and correlation coefficients at different stirring speeds

3.5 Characterization of residue
The residue, which reacted for 30 min under the optimized conditions of leaching temperature 90 °C, mass ratio of NaOH to SiO2 2:1 and stirring speed 300 r/min, was characterized by chemical analysis, XRD pattern and SEM image.
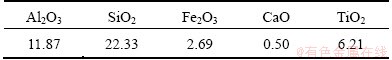
The chemical analysis of residue is shown in Table 6. Compared with Al2O3 extracted slag, the SiO2 content in the residue is lowered while the Al2O3 content is improved. From Table 6, it can be seen that only 22.33% of SiO2 remains in the residue. WU et al [19] studied extracting Al2O3 from fly ash by acid method, and then investigated the dissolving behavior of SiO2 in Al2O3 extracted slag with high concentrated NaOH solution. In their work, the mass fraction of SiO2 in residue was 37.79%. Thereby, SiO2 leaching effect is better by the present method.
Table 6 Composition of residue (mass fraction, %)
The XRD pattern of residue is shown in Fig. 13. As illustrated in Fig. 13, there is no mineral phase of sodium-silicon slag, indicating that the SiO2 leaching reaction is efficient and less SiO2 is lost under the optimized conditions. Additionally, Fig. 13 also shows that the glass phase disappears, whereas, α-SiO2 phase is still there. This result manifests that the reaction activity of glass phase with NaOH is higher than that of α-SiO2.
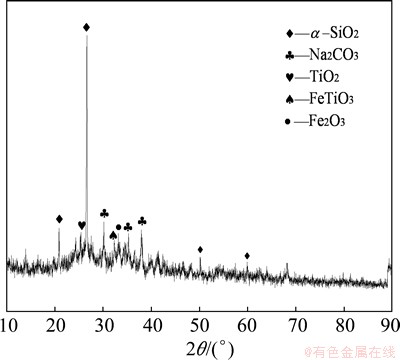
Fig. 13 XRD pattern of residue
The morphology of residue was examined by SEM as shown in Fig. 14. It can be seen that the particles of Al2O3 extracted slag were almost completely broken after NaOH leaching.

Fig. 14 SEM image of residue
4 Conclusions
1) The effect of leaching temperature, mass ratio of NaOH to SiO2 and stirring speed on SiO2 leaching rate from Al2O3 extracted slag is noticeable. The SiO2 leaching rate increases with increasing the leaching temperature, mass ratio of NaOH to SiO2 and stirring speed. The SiO2 leaching rate is up to 95.66% under the optimized conditions of leaching temperature 90 °C, mass ratio of NaOH to SiO2 2:1, stirring speed 300 r/min and leaching time 30 min.
2) The leaching process can be divided into two stages: 1-5 min is the first stage; 5-30 min is the second stage. The leaching reaction is very rapid in the first stage but quite slow in the second stage. The whole leaching process follows the shrinking core model, and the outer diffusion of no product layer is the rate-controlling step. The activation energies of the first and second stages are calculated to be 8.492 kJ/mol and 8.668 kJ/mol, respectively. The kinetic equations of the first and second stages can be expressed as 1-(1-α)2/3=0.5409exp[-8492/(RT)]t and 1-(1-α)2/3= 0.046exp[-8668/(RT)]t, respectively.
3) After NH4HSO4 roasting of fly ash, the Al2O3 extracted slag was used as raw material to recover SiO2 with NaOH solution. Compared with other methods, the SiO2 leaching process is more efficient in this work, which has many merits of convenient for recycling SiO2, less energy consumption and low cost.
References
[1] SWANEPOEL J C, STRYDOM C A. Utilisation of fly ash in a geopolymeric material [J]. Applied Geochemistry, 2002, 17(8): 1143-1148.
[2] QUEROL X, MORENO N,  J C, ALASTUEY A,
J C, ALASTUEY A,  A, PLANA F. Synthesis of zeolites from coal fly ash: An overview [J]. International Journal of Coal Geology, 2002, 50(1-4): 413-423.
A, PLANA F. Synthesis of zeolites from coal fly ash: An overview [J]. International Journal of Coal Geology, 2002, 50(1-4): 413-423.
[3] IYER R S, SCOTT J A. Power station fly ash—A review of value-added utilization outside of the construction industry [J]. Resources, Conservation and Recycling, 2001(3), 31: 217-228.
[4] RAYZMAN V L, SHCHERBAN S A, DWORKIN R S. Technology for chemical-metallurgical coal ash utilization [J]. Energy & Fuels, 1997, 11(4): 761-773.
[5] PADILLA R, SOHN H Y. Sodium aluminate leaching and desilication in lime soda sinter process for alumina from coal wastes [J]. Metallurgical and Materials Transaction B, 1985, 16(4): 707-713.
[6] SEIDEL A, ZIMMELS Y. Mechanism and kinetics of aluminum and iron leaching from coal fly ash by sulfuric acid [J]. Chemical Engineering Science, 1998, 53(22): 3835-3852.
[7] DUTTA B K, KHANRA S. Leaching of elements from coal fly ash: Assessment of its potential for use in filling abandoned coal mines [J]. Fuel, 2009, 88(7): 1314-1323.
[8] WU Cheng-you, YU Hong-fa, ZHANG Hui-fang. Extraction of aluminum by pressure acid-leaching method from coal fly ash [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(9): 2282-2288.
[9] SEIDEL A, SLUSZNY A, SHELEF G, ZIMMELS Y. Self inhibition of aluminum leaching from coal fly ash by sulfuric acid [J]. Chemical Engineering Journal, 1999, 72(3): 195-207.
[10] KELMERS A D, CANON R M, EGAN B Z, FELKER L K, GILLIAM T M, JONES G, OWEN G D, SEELEY F G, WATSON J S. Chemistry of the direct acid leach, calsinter, and pressure digestion-acid leach methods for the recovery of alumina from fly ash [J]. Resources and Conservation, 1982, 9: 271-279.
[11] MATJIE R H, BUNT J R, van HEERDEN J H P. Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal [J]. Minerals Engineering, 2005, 18(3): 299-310.
[12] WANG Jia-dong, ZHAI Yu-chun, SHEN Xiao-yi. Study on extracting silica from fly ash by alkali leaching [J]. Light Metals, 2008(12): 23-25. (in Chinese)
[13] WANG Jia-dong, ZHAI Yu-chun, SHEN Xiao-yi. Extracting Al2O3 from desiliconized fly ash with alkali lime sintering process [J]. Light Metals, 2009(6): 14-15. (in Chinese)
[14] WU Yan. Study on extraction of Al2O3 and SiO2 from fly ash [D]. Shenyang: Northeastern University, 2008: 64-76. (in Chinese)
[15] LI Hong-gui. Metallurgical principles [M]. Beijing: Science Press, 2012: 291-316. (in Chinese)
[16] LI Hong-gui. Hydrometallurgy [M]. Changsha: Central South University Press, 2002: 69-100. (in Chinese)
[17] LI Xiao-bin, QI Tian-gui, PENG Zhi-hong, ZHOU Qiu-sheng. Kinetics of chromite ore in oxidation roasting process [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(9): 1822-1828. (in Chinese)
[18] HUA Yi-xin. Introduction of metallurgical process kinetics [M]. Beijing: Metallurgical Industry Press, 2004: 191-193. (in Chinese)
[19] WU Yan, ZHAI Yu-chun, MU Wen-ning, SUN Yang. Dissolving behavior of SiO2 in residue of fly ash detached Al2O3 in highly concentrated alkali solution [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(1): 407-411. (in Chinese).
粉煤灰提铝渣碱浸提硅动力学
王若超,翟玉春,宁志强,马培华
东北大学 材料与冶金学院,沈阳 110819
摘 要:对粉煤灰提铝渣碱浸提硅过程的动力学进行研究。实验考察碱浸温度、碱硅比(NaOH与SiO2质量比)及搅拌速度对二氧化硅浸出率的影响。实验结果表明,升高碱浸温度、加大碱硅比及加强搅拌速度对浸出过程有利,在最优条件下,二氧化硅浸出率为95.66%。该浸出过程分为两个阶段,在第一阶段二氧化硅的浸出速率快,在第二阶段二氧化硅的浸出速率慢,两阶段均符合收缩核模型且受不生成固体产物层的外扩散控制。碱浸提硅过程第一阶段和第二阶段的反应活化能分别为8.492 kJ/mol和8.668 kJ/mol,分别求得这两个阶段的动力学方程。
关键词:粉煤灰;浸出;动力学;二氧化硅
(Edited by Hua YANG)
Foundation item: Project (2007CB613603) supported by the National Basic Research Program of China; Project (2013M530934) supported by the Postdoctoral Science Foundation of China
Corresponding author: Yu-chun ZHAI; Tel: +86-13709845210; E-mail: 370347814@163.com
DOI: 10.1016/S1003-6326(14)63273-8
Abstract: Kinetics of SiO2 leaching from Al2O3 extracted slag of fly ash with sodium hydroxide solution was studied. The effect of leaching temperature, mass ratio of NaOH to SiO2 and stirring speed on SiO2 leaching rate was investigated. The results show that increasing leaching temperature, mass ratio of NaOH to SiO2 and stirring speed increases SiO2 leaching rate. The SiO2 leaching rate is 95.66% under the optimized conditions. There are two stages for the SiO2 leaching process, and the leaching reaction is very rapid in the first stage but quite slow in the second stage. The whole leaching process follows the shrinking core model, and the outer diffusion of no product layer is the rate-controlling step. The activation energies of the first and second stages are calculated to be 8.492 kJ/mol and 8.668 kJ/mol, respectively. The kinetic equations of the first and the second stages were obtained, respectively.


