J. Cent. South Univ. (2016) 23: 2321-2328
DOI: 10.1007/s11771-016-3290-y

Experiment study of optimization on prediction index gases of coal spontaneous combustion
NIU Hui-yong(牛会永)1, 2, DENG Xiang-ling(邓湘陵)1, LI Shi-lin(李石林)1, 2, CAI Kang-xu(蔡康旭)1, 2,
ZHU Hao(朱豪)1, LI Fang(李芳)1, DENG Jun(邓军)1
1. School of Energy and Safety Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
2. Hunan Provincial Key Laboratory of Safe Mining Techniques of Coal Mines (Hunan University of Science and
Technology), Xiangtan 411201, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
The coal of Anyuan Mine has the characteristic of easy spontaneous combustion. Conventional method is difficult to predict it. Coal samples from this mine were tested in laboratory. The data obtained from laboratory determination were initialized for the value which was defined as “K”. The ratio of each index gas and value of “K”, and the ratio of combination index gases and value of “K”, were analyzed simultaneously. The research results show that for this coal mine, if there is carbon monoxide in the gas sample, the phenomenon of oxidation and temperature rising for coal exists in this mine; if there is C2H4 in the gas sample, the temperature of coal perhaps exceeds 130 °C. If the coal temperature is between 35 °C and 130 °C, prediction and forecast for coal spontaneous combustion depend on the value of Φ(CO)/K mainly; if the temperature of coal is between 130 °C and 300 °C, prediction and forecast for coal spontaneous combustion depend on the value of Φ(C2H6)/Φ(C2H2) and Φ(C2H6)/K. The research results provide experimental basis for the prediction of coal spontaneous combustion in Anyuan coal mine, and have better guidance on safe production of this coal mine.
Key words:
coal; spontaneous combustion; index gases; prediction; initialization;
1 Introduction
Coal spontaneous combustion is one of the main disasters in coal mine. If characteristics of coal spontaneous combustion can be measured in advance, and coal oxidation conditions can be predicted accurately, the implementation of preventive measures for coal mine fire would be made effectively. In general, through analyzing the prediction results of coal spontaneous combustion, change rules of different parameters in the process of coal spontaneous combustion could be summed up [1-4]. The relations between coal temperature and index gases concentration could be ascertained. The degree of coal oxidation and its changes could be judged correctly. At present, method of analyzing the index gases [5-9], method of the temperature [10-13] and the simulation method [14-18], have been used for predicting coal spontaneous combustion usually. The research about predicting coal spontaneous combustion is mainly concentrated in the selection of index gases, construction of prediction model and the process simulation of coal spontaneous combustion. The research on the prediction model is limited to comparison between theoretical curve and actual curve [19-26]. In the above research methods, analyzing index gases is widely applied to the spontaneous combustion forecast for coal mine safety production, and it is used to forecast the degree of coal oxidation and its changes. However, in the practical application, the temperature of coal predicted by the experimental model often has a large deviation with the actual coal temperature, which will directly affect the effect of the mine fire prevention. When the quality of coal is certain, the type of gas generated from coal and the concentration and temperature have a certain rule. Therefore, according to the mine actual observation conditions, through analyzing the relationship between temperatures of gas concentration, the certain rule can be found to predict the degree of coal spontaneous combustion in earlier stage. The method of index gases is widely used in coal mine. In this work, through using the experimental device which was set up by author’s research team, the relationship between different index gases in the process of coal spontaneous combustion was analyzed. Based on the theory and technology of forecast model developed by author’s research team, the experimental analysis and the forecast for coal spontaneous combustion were carried out, which could solve the difficult problem of coal spontaneous combustion in Anyuan mine.
2 Theory and methods of initialization
In this work, the concentration of gas components released in the process of coal spontaneous combustion of coal was initialized, and the problem of false alarm for coal spontaneous combustion was solved. To understand the detailed theoretical analysis of this method, readers can read the research results [27-30] from author’s research team. In order to ensure the consistency for the reader to understand this article, this technology processing method is briefly induced as follows.
2.1 Heat transfer equation in process of coal combustion
Coal can be compared to porous media, and heat transfer process of porous media is extremely complicated. Experiments and theory-analysis indicate that radiation thermal effect is just considered in high vacuum and high temperature, but ignored in normal condition. The size of pores in porous media is small, which can prevent convection. The quantity of heat convection of liquid in pores has little effect and it can be ignored. Generally, heat transfer process of porous media is seemed as heat conduction of solid and liquid. The thermal conductivity between solid and liquid is different because of their physical properties. In normal pressure, thermal contact resistance among solid particles can be neglected. In low pressure and mid temperature, thermal contact resistance cannot be overlooked.
If radiation heat transfer is neglected, the solid particles in micro-body and the gas in pores obey the law of energy conservation.
As for coal:
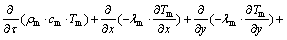
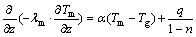 (1)
(1)
where n represents voidage of coal; Tm represents coal temperature, °C; Tg represents average temperature of liquid, °C; α represents coefficient of convection heat transfer, J/(°C·s·cm2); cm represents specific heat capacity of coal, J/(g·°C); ρm represents density of coal, g/cm3; q represents calorific value of per unit loose coal, J/cm3; τ represents time, s.
The above formula can be represented by the following formula, this is:
 (2)
(2)
As for liquid:
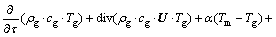
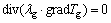 (3)
(3)
where cg represents specific heat capacity of liquid, J/(g·°C); ρg represents density of liquid, g/cm3; U represents velocity of liquid, cm/s.
Velocity of seepage gas in loose coal is commonly low and solid particles are almost considered homogenization and isotropism; then λ, ρ, cg and cm do not change with coordinates. We can consider that Tm and Tg are identical and they are seemed as T. The two previously mentioned formulas can be combined:
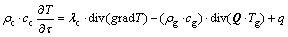 (4)
(4)
where ρc represents equivalent gross density of loose coal, 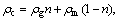 g/cm3; cc represents equivalent specific heat capacity of loose coal, cc=cgn+ cm(1-n), J/(g·°C); λc represents coefficient of thermal conductivity of loose coal, J/(m·s·°C); Q represents air leak intensity, Q=n·U, cm3/(cm2·s).
g/cm3; cc represents equivalent specific heat capacity of loose coal, cc=cgn+ cm(1-n), J/(g·°C); λc represents coefficient of thermal conductivity of loose coal, J/(m·s·°C); Q represents air leak intensity, Q=n·U, cm3/(cm2·s).
From the above formula, the requirement of loose coal temperature raised in mine:

 (5)
(5)
2.2 Reason of initialization
The ratio of Φ(CO) to Φ(CO2), is the main parameter for coal temperature prediction during the slow temperature oxidation of coal (35-150 °C). However, there are two problems in practical application.
1) The existence of CO2 is very popular in coal mine; furthermore, initial concentration of CO2 is also different in different locations (such as roadway, top-coal falling region, goaf, sealed area). Difference of concentration can vary from a few times to more than ten times and it is bound to cause a large prediction error in many locations.
2) In the early stage of spontaneous combustion of coal, the amount of CO is much less than that of CO2, and the value of Φ(CO)/Φ(CO2) is very small (see Table 1), which is not convenient for the worker to analyze correctly.
2.3 Method of initialization process
The initialization process means that, the error of temperature caused by different points, can be excluded by normalizing the value of Φ(CO2) under room temperature. Therefore, results of spontaneouscombustion prediction by using Φ(CO2) at different locations can be compared under the same standard, and these values can be read and processed easily and accurately.
Table 1 Comparison of treatment methods for CO2

Initialization process is calculated as K=A/r. In this formula, K represents initialized value; A=Φ(CO)+ Φ(CO2), and the value of Φ(CO)+Φ(CO2) can moderate numerical oscillations of Φ(CO) and Φ(CO2) caused by the variation of oxygen; r represents the initialization constant and the value of r is as volume fraction of CO2 (concentration of CO2 when CO can be found under room temperature, the location is different and this value is not the same) in room temperature.
After initialization, different kinds of locations have the relatively consistent starting point (means K=1). These values of Φ(CO)/K are larger than 1, thus, values can be identified easily and compared each other. When the value of Φ(CO)/K is used as the calculation model, it is effective to avoid the error of prediction caused by the different initial value of Φ(CO2) at different areas at room temperature and different air volumes.
3 Experimental procedure
3.1 Coal sample collection and disposition
Anyuan Coal Mine is located in Anyuan town, Southeast of Pingxiang city, Jiangxi Province. The mining coal seam has the tendency of ignition, and the spontaneous ignition period is about 3 months to 6 months. Anyuan Coal mine belongs to the Second Kind of coal mine spontaneous combustion. In this experiment, skillful workers collected eight coal samples along coal seam trend in Dacao Coal seam. Each coal sample was not less than 2 kg, with double plastic bags. Samples were machined by ball mill and mesh screen.
3.2 Experiment system
The test system was established by my research team. It was mainly composed of gas chromatograph, data processing system and coal sample heating system. In order to test several coal samples at the same time, Multiple Storehouse Program Control Heating Device was designed by research team members, including multiple storehouse heating furnace, micro pump, flow meter, temperature control program, as shown in Fig. 1.
In order to ensure that furnace temperature (this temperature is usually higher than the temperature in sample storehouse) and the temperatures of the sample are equal, to ensure that the coal in sample storehouse can be heated uniformly, to improve the reliability of comparison among gases produced from samples in each sample storehouse, this heating device was established with a double thermometer. Four samples can be put into sample storehouse at the same time, the mass of each coal is 1 g, and the sample coal can be heated to 600 °C.

Fig. 1 Schematic diagram of multiple storehouse program control heating device
3.3 Experiment parameters and process
3.3.1 Selection of particle size of coal sample and gas flow
Through testing various particle sizes, such as more than 180 μm, 150-180 μm, less than 150 μm, it was proved that the ability to take oxygen of the coal sample with the particle size of less than 150 μm was the strongest. Therefore, coal samples less than 150 μm, were selected as experimental sample in the process of coal spontaneous combustion test. In the process of coal sample heating, 50 mL/min was selected as the air flow rate, and the stability of the air flow was maintained.
3.3.2 Controlling oxidation heating rate of coal
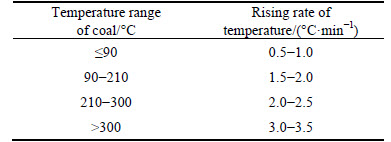
Usually, oxidation heating rate of coal is slow when the coal sample is in initial stage (below 100 °C) of oxidation; oxidation heating rate of coal begin to speed up when it is in middle stage (100-200 °C) of oxidation; oxidation heating rate of coal is becoming more and more rapid when it is in late stage (above 200 °C) of oxidation. Based on this, the temperature rising speed control program is divided into four stages in the course of this experiment, as listed in Table 2.
Table 2 Control parameters of coal heating rate
3.3.3 Temperature interval for taking gas sample and type of gas
The time of chromatographic analysis is balanced with the rising rate of temperature of coal, as listed in Table 3.
According to the actual situation of Anyuan coal mine, two types of gases will be tested in laboratory: conventional gases include O2, N2, CO, CO2 and CH4; multi hydrocarbon gases include C2H6, C2H4, C2H2, C3H8,C3H6 and C4H10,
Table 3 Temperature interval for taking gas sample

4 Results and discussion
4.1 Determination of index gases in Anyuan coal mine
According to the experimental results, index gases, produced by Dacao coal sample about at 40 °C, include CO, C2H2, C3H8 and C4H10; C2H6 appears at 45-130 °C and the average temperature is 60 °C; C2H4 appears at 90-130 °C and the average temperature is 110 °C; C3H6 appears at 70-150 °C and the average temperature is 130 °C; C4H10 appears at 45- temperature of the sample are 210 °C and the average temperature is 110 °C.
Through analyzing data, characteristics of the index gases generated from Dacao coal in Anyuan coal mine has been summarized as follows.
1) Property of room temperature: Coal can generate C2H2 usually only at high temperature; however, C2H2 can be detected at Dacao coal seam under room temperature in Anyuan coal mine. Therefore, C2H2 lost the role of judging the state of coal.
2) Property of sudden increase: When the temperature of coal is about in 170-200 °C, due to the sudden increase in the amount of oxygen consumed by the coal (oxidation acceleration), the amount of index gases generated from coal will also increase by 3-10 times, or even more. A large number of explosive gases, such as CO and CH4, increase the risk of rescuing mine fire. If a large number of coals have been oxidized in the higher temperature zone, the concentration of flammable gas will reach the explosion limit quickly, and makes rescue man in danger.
3) Multi gas species: There are more than 15 kinds of CmHn gases produced by the coal sample in Anyuan coal mine. Because the tail of the peak (invalid peak after effective peak appears) continues to appear in 15-20 min, this phenomenon makes gas sample require more time to be analyzed. Moreover, the effective peak is easy to be interfered, and the data cannot be read correctly.
4.2 Characteristic analysis of index gas
4.2.1 Characteristics analysis of various individual index gas
First of all, characteristics analysis of various individual index gas in Anyuan coal mine, are focused on CO, CO2, C2H6, C2H4 and C2H2. Each kind of index gas is generated form eight different areas, and the average value is calculated. Their changes of volume fraction with temperature are shown in Fig. 2 and Fig. 3.
Curves of Φ(CO) and Φ(CO2) are standard within 270 °C and they show placid within 130 °C. However, CO has many shortcomings in predicting coal spontaneous combustion and the temperature detection range of CO is extremely wide. The relationship between the amounts of Φ(CO) and coal temperature, is not clear. Especially, under complex actual conditions in mine, the value of Φ(CO) can be affected by environmental factors easily. Therefore, it is difficult to ascertain the stage of coal spontaneous combustion through analyzing the concentration of CO, and decreases the prediction accuracy and precision. CO2 appears at 30 °C, and the amount of Φ(CO2) is larger. Therefore, the value of Φ(CO2) cannot be seemed as index gas singly. The curve of Φ(C2H6) is placid within 150 °C, and there is no law after 150 °C. The values of Φ(C2H4) are zero within 90 °C, and are very small within 110-130 °C; shape of Φ(C2H4) curve likes saddle within 130-360 °C and its peak is more discrete. The curve of Φ(C2H2) is more standard. However, coal samples in Anyuan mine are different from other mines. C2H2 can be generated at room temperature, and the whole curve is similar to Gaussian distribution; the vertex of the curve appears at 170 °C. Therefore, C2H2 cannot be seemed as index gas for predicting coal spontaneous combustion. The concentration of C2H4 with the change of temperature is relatively standard and it can be used for gas analysis for its sensitive index sign in coal fire. However, the value Φ(C2H4) is very small and the sensitivity of detection and prediction can be affected easily.
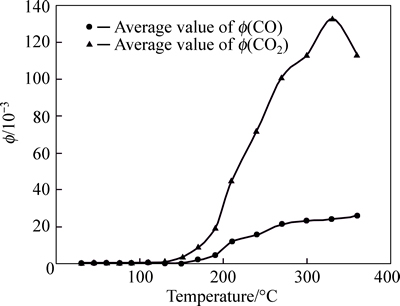
Fig. 2 Tendency charts of Φ(CO) and Φ(CO2) in Anyuan coal mine
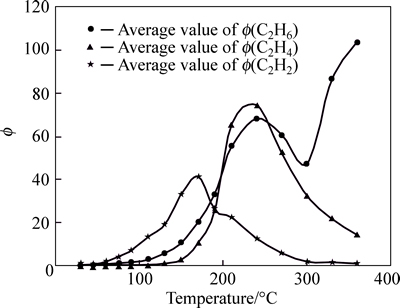
Fig. 3 Tendency chart of Φ(C2H6), Φ(C2H4) and Φ(C2H2) in Anyuan coal mine
4.2.2 Characteristics analysis of various index gases composition
In order to further take the regularity of index gases, and reveal the characteristics of the coal spontaneous combustion in Anyuan coal mine, the author analyzes these index gases combination, concluding Φ(CO)+ Φ(CO2) and Φ(C2H6)+Φ(C2H4). Tendencies are shown in Fig. 4 and Fig. 5.

Fig. 4 Tendency chart of Φ(CO)+Φ(CO2) in Anyuan Coal Mine
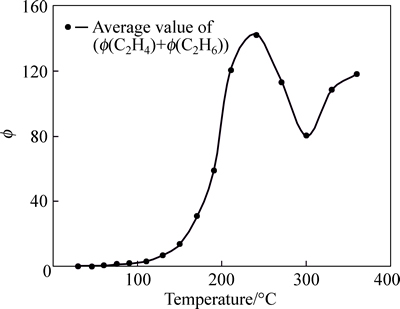
Fig. 5 Tendency chart of Φ(C2H6)+Φ(C2H4) in Anyuan Coal Mine
Curves of the combined item show that, curve of Φ(CO)+Φ(CO2) is standard in whole process, and it is placid within 130 °C. This curve cannot accurately reflect the change state of coal temperature, and the content of CO2 is larger than that of CO. Therefore, the value of Φ(CO)+Φ(CO2) should not be used as index gas. However, curve of Φ(C2H6)+Φ(C2H4) is discrete after 130 °C, and it appears worse regularity. It cannot be used as index gas also.
According to the above analysis, for Dacao coal seam in Anyuan mine, spontaneous combustion of the coal seam cannot be predicted through analyzing the single gas sample or combined item only.
4.3 Analysis of relationship between ratio of index gases and coal temperature
4.3.1 Initialization of Φ(CO)+Φ(CO2)
According to the method of initialization processing described above, dates of index gases taken in the experimental are initialized. “K” represents the ratio of A (A=Φ(CO)+Φ(CO2)) value from different areas and initial Φ(CO2) value (value at room temperature in coal mine), that is K=A/r.
Table 4 Initialization for value of “K” in Dacao coal seam

4.3.2 Analysis of ratio curves among index gases
Ratio values among index gases are analyzed. These values include Φ(C2H4)/Φ(C2H6), Φ(C2H6)/Φ(C2H2), Φ(C2H6)/K, Φ(CO)/K and Φ(C2H4)+Φ(C2H6)/K. Ratio curves are shown in Figs. 6-9.
Curve of Φ(C2H4)/Φ(C2H6) is not standard within 210 °C; however, this curve appears certain regularity after 210 °C. The curve of Φ(C2H4)/Φ(C2H6) can be used as supplement to other index gas. Gaussian distribution appears within 240 °C for Φ(C2H6)/K, except the discretion for few sample. The curve of Φ(C2H6)/K is stabile after 240 °C. Therefore, in the late stage of coal spontaneous combustion, Φ(C2H6)/K can be chosen as a criterion for predicting coal fire. The curve of Φ(CO)/K is standard within 30-210 °C, and its sensitivity is better; when temperature exceeds 210 °C, the curve is volatile, although they tend to be gentle. Therefore, in the early stage of coal spontaneous combustion, Φ(CO)/K can be chosen as the index gas to predict coal temperature. The whole curve of (Φ(C2H4)+Φ(C2H6))/K appears Gaussian distributions and the vertex of the curve appears within 130-170 °C. This curve is stable after 210 °C. Although there is certain regularity for (Φ(C2H4)+Φ(C2H6))/K, due to its small value, error often occurs in process of analyzing the data, and the temperature of coal is incorrect; Therefore, this curve is not used as index gas also. The curve of Φ(C2H6)/Φ(C2H2) shows that, the change of coal temperature is not obvious in low temperature phase, but it is very sensitive in the high temperature phase. Therefore, when the temperature of the coal exceeds 130 °C, this parameter can be used as main index gas.
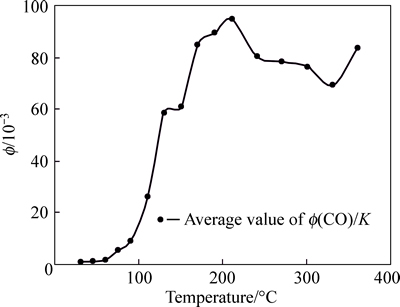
Fig. 6 Tendency chart of Φ(CO)/K in Anyuan coal mine
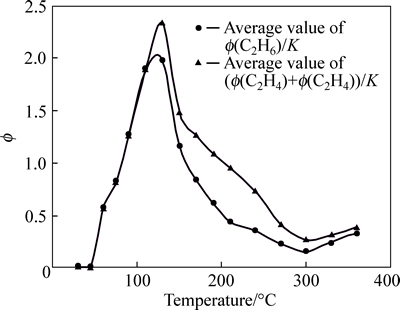
Fig. 7 Tendency charts of (Φ(C2H4)+Φ(C2H6))/K and Φ(C2H6)/K in Anyuan Coal Mine
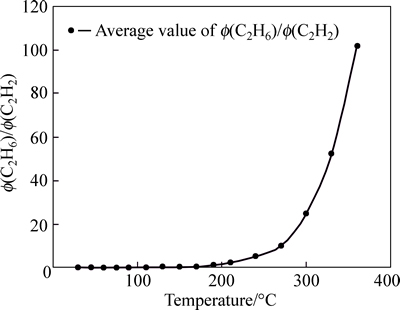
Fig. 8 Tendency chart of Φ(C2H6)/Φ(C2H2) in Anyuan Coal Mine
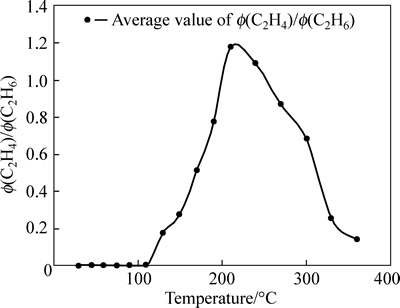
Fig. 9 Tendency chart of Φ(C2H4)/Φ(C2H6) in Anyuan Coal Mine
4.4 Prediction standard of spontaneous combustion in Anyuan coal mine and related rescue measures
4.4.1 Procedure of prediction
1) Because of the lower temperature which can make the coal in Anyuan Mine release CO, if CO can be detected in gas sample, the temperature of the coal is considered to be 35 °C nearly, and coal is being oxidized. According to judgment conditions, the calculation and prediction are based on the ratio of Φ(CO)/K in the range of 35-130 °C.
2) If C2H4 can be detected in gas sample, the temperature of the coal is considered to be above 130 °C. The calculation and prediction are based on the ratio of Φ(C2H6)/Φ(C2H2) and Φ(C2H6)/K in the range of 130-300 °C.
4.4.2 Division of coal oxidation stage
Based on the calculation of coal temperature, the process of coal spontaneous combustion is divided into three stages: the slow oxidation stage, the accelerated oxidation stage and the intense oxidation stage.
The slow oxidation stage: In this stage, the temperature of coal is 35-130 °C; workers in mine should strengthen the observation to find out the high temperature point, and should take effective cooling measures;
The accelerated oxidation stage: In this stage, the temperature of coal is 130-300 °C, coal mining should be stopped, and material for sealing fire area should be ready for grouting;
The intense oxidation stage: In this stage, the temperature of coal is above 300 °C and leaders of this coal mine should organize workers to leave, and the fire area should be sealed and grouted rightly.
4.4.3 Three key points for Anyuan coal mine
By applying prediction model, and analyzing the relationship between the temperature of coal predicted in laboratory and the temperature under Anyuan coal mine, three key points are determined as follows: CO appears at 35 °C in Anyuan mine, or C2H6 appears at 60 °C; C2H4 appears at 130 °C; coal in Anyuan mine will not appear to smoke at 300 °C.
5 Conclusions
1) Although CO is often used in many coal mines because of its standard curve, under complicated conditions in coal mine, the method of using Φ(CO) curves is affected by airflow, primary gases in coal, selection of detect, process of production, and other factors. Therefore, the accuracy and the precision of prediction decrease. The prediction of coal spontaneous combustion is not ideal through analyzing gas index. However, combination or ratio among gas indexes is an effective method for prediction.
2) The value of “K”, proposed in this work, can eliminate the influence of environmental factors on gas indexes. Results show that these curves (the ratio of each gas index and value of “K”, and ratio of combined gas index and value of “K”) can forecast coal spontaneous combustion. In the whole process of prediction, according to various sensitivity of gas indexes in different temperature ranges, interval prediction can be implemented in Anyuan coal mine.
3) Based on experiment and actual conditions in Anyuan coal mine, coal spontaneous combustion prediction curves of index gases are put forward. Rescue of preventing coal mine fire is drawn up for mine.
References
[1] XIAO Yang, MA Li, WANG Zhen-ping, WEN Hu, DENG Jun. Law of index gases adsorption and condensability of coal spontaneous combustion [J]. Journal of China Coal Society, 2007, 32(10): 1014-1018. (in Chinese)
[2] DENG Jun, LI Bei, LI Zhen-bao, ZHANG Ying, GUAN Xin-jie. Experiment study on gas indexes optimization for coal spontaneous combustion prediction [J]. Coal Science and Technology, 2014, 42(1): 55-59, 79. (in Chinese)
[3] JIANG Shu-guang, ZHANG Wei-qing, WANG Lan-yun, WU Zheng-yan, CHEN Yue-qing, LIANG Wei-wei, HAO Quan. B-mode relational degree analysis of index gases in the high-temperature section of coal spontaneous combustion [J]. Journal of Mining & Safety Engineering, 2009, 26(3): 377-380. (in Chinese)
[4] GIL M V,  R, PEVIDA C, RUBIERA F. Grindability and combustion behavior of coal and torrefied biomass blends [J]. Bioresource Technology, 2015, 191(9): 205-212.
R, PEVIDA C, RUBIERA F. Grindability and combustion behavior of coal and torrefied biomass blends [J]. Bioresource Technology, 2015, 191(9): 205-212.
[5] DENG Jun, ZHAO Jing-yu, ZHANG Yan-ni. Study on determination of coal spontaneous combustion characteristic temperature based on analysis method of index gas growth-rate [J]. Coal Science and Technology, 2014, 42(7): 49-52, 56. (in Chinese)
[6] TAN Bo, HU Rui-li, GAO Peng, LI Kai. Experimental study on stage characteristics of coal spontaneous combustion disaster gas indicators [J]. China Safety Science Journal, 2013, 23(2): 51-57. (in Chinese)
[7] LIU Qiao, WANG De-ming, ZHONG Xiao-xing, JIAO Xin-ming, ZHANG Hui-jun. Testing on indicator gases of coal spontaneous combustion based on temperature program [J]. Journal of Liaoning Technical University: Natural Science, 2013, 32(3): 362-366. (in Chinese)
[8] HU Xin-cheng, YANG Sheng-qiang, ZHOU Xiu-hong, YU Zhao- yang, HU Chun-ya. Coal spontaneous combustion prediction in gob using chaos analysis on gas indicators from upper tunnel [J]. Journal of Natural Gas Science and Engineering, 2015, 26(9): 461-469.
[9] DENG Jun, XIAO Yang, LI Qing-wei, LU Jun-hui, WEN Hu. Experimental studies of spontaneous combustion and anaerobic cooling of coal [J]. Fuel, 2015, 157(1): 261-269.
[10] ZHU Hong-qing, WANG Hai-yan, WANG Fei-ran, YANG Cheng-yi. Research progress on coal stockpile temperature measuring technology [J]. Coal Science and Technology, 2014, 42(1): 50-54. (in Chinese)
[11] WEN Hu, WU Kang, MA Li, WANG Wei-feng, WANG Tao. Application of distributed optical fiber temperature measurement system in monitoring goaf coal spontaneous combustion [J]. Safety of Coal Mines, 2014, 45(5): 100-102, 105. (in Chinese)
[12] ZHU Jian-fang, HE Ning, LI Deng-ji. The relationship between oxygen consumption rate and temperature during coal spontaneous combustion [J]. Safety Science, 2012, 50(4): 842-845.
[13] YUAN Li-ming, SMITH A C. The effect of ventilation on spontaneous heating of coal [J]. Journal of Loss Prevention in the Process Industries, 2012, 25(1): 131-137.
[14] LIU Xing-kui, YANG Shu-zhao. Numerical simulation of heating up and air leakage distribution for spontaneous combustion of coal pile [J]. Journal of Henan Polytechnic University: Natural Science, 2015, 34(5): 610-614. (in Chinese)
[15] ZHOU Yan, MENG Qian, LI Jun, LIN Bai-quan, XU Cong. Research on numerical simulation for spontaneous combustion zone in gob area [J]. Journal of China University of Mining and Technology, 2014, 43(6): 963-968. (in Chinese)
[16] TARABA B, PAVELEK Z. Study of coal oxidation behaviour in re-opened sealed heating [J]. Journal of Loss Prevention in the Process Industries, 2016, 40(3): 433-436.
[17] ZHANG Chun, TI Zheng-yi, LI Zong-xiang. Three-dimension heterogeneity dynamic numerical simulation of top coal spontaneous combustion in limit equilibrium zone [J]. China Safety Science Journal, 2012, 22(5): 37-43. (in Chinese)
[18] YILDIRIM O S, SENSOGUT C, GOKAY M K. Effects of electrical resistance on the spontaneous combustion tendency of coal and the interaction matrix concept [J]. Journal of University of Science and Technology Beijing: Mineral, Metallurgy, Material, 2006, 13(1): 1-6. (in Chinese)
[19] JIN Yong-fei, ZHAO Rui-yuan, DENG Jun, LIU Wen-yong, FEI Jin-biao, GUO Jun. Experiment study on multi parameters prediction indexes of coal spontaneous combustion [J]. Coal Science and Technology, 2014, 42(9): 112-114, 76. (in Chinese)
[20] CHU Ting-xiang, YU Ming-gao, LI Long-fei. Identification of information on spontaneous combustion environment for coal in goaf and determination of forecast indexes [J]. China Safety Science Journal, 2014, 24(8): 151-157. (in Chinese)
[21] SAHU H B, MAHAPATRA S S, SIRIKASEMSUK K, PANIGRAHI D C. A discrete particle swarm optimization approach for classification of Indian coal seams with respect to their spontaneous combustion susceptibility [J]. Fuel Processing Technology, 2011, 92(3): 479-485.
[22] XU Yong-liang, WANG De-ming, WANG Lan-yun, ZHONG Xiao-xing, CHU Ting-xiang. Experimental research on inhibition performances of the sand-suspended colloid for coal spontaneous combustion [J]. Safety Science, 2012, 50(4): 822-827.
[23] ZHANG Jian-an, LI Wen-jun, LIU Han-yong, WANG An-liang. Forecast of spontaneous combustion fire in goaf based on rough set and cluster [J]. Journal of Xi’an University of Science and Technology, 2012, 32(6): 696-701. (in Chinese)
[24] YUAN Li-ming, SMITH A C. CO and CO2 emissions from spontaneous heating of coal under different ventilation rates [J]. International Journal of Coal Geology, 2011, 88(1): 24-30.
[25] ROSEMA A, GUAN H, VELD H. Simulation of spontaneous combustion, to study the causes of coal fires in the Rujigou Basin [J]. Fuel, 2001, 80(1): 7-16.
[26] LI Bo, CHEN Gang, ZHANG Hui, SHENG Chang-dong. Development of non-isothermal TGA–DSC for kinetics analysis of low temperature coal oxidation prior to ignition [J]. Fuel, 2014, 118(2): 385-391.
[27] CAI Kang-xu, YUAN Qiang, OUYANG Dong-hua, LIU Ai-hua. Spontaneous combustion and forecast method for high-sulfur coal [J]. Mineral Engineering Research, 2009, 24(3): 41-44. (in Chinese)
[28] CAI Kang-xu, CEN Dai-quan, YUAN Qiang, LIU Ai-hua. Correction theory and method of the coal spontaneous combustion forecasting model [J]. Mineral Engineering Research, 2009, 24(1): 63-67. (in Chinese)
[29] LIU Ai-hua, CAI Kang-xu. Study on predicting coal spontaneous combustion and software development [J]. Journal of China Coal Society, 2007, 32(7): 724-728. (in Chinese)
[30] LIU Ai-hua, CAI Kang-xu. Research on identification gas for coal spontaneous combustion in Qingshan mine [J]. Coal Science and Technology, 2006, 34(7): 11-13, 16. (in Chinese)
(Edited by YANG Hua)
Foundation item: Projects(51274099, 51474106) supported by the National Natural Science Foundation of China
Received date: 2016-04-05; Accepted date: 2016-07-08
Corresponding author: NIU Hui-yong, Associate Professor, PhD; Tel: +86-13789303026; E-mail: niuhuiyong@163.com
Abstract: The coal of Anyuan Mine has the characteristic of easy spontaneous combustion. Conventional method is difficult to predict it. Coal samples from this mine were tested in laboratory. The data obtained from laboratory determination were initialized for the value which was defined as “K”. The ratio of each index gas and value of “K”, and the ratio of combination index gases and value of “K”, were analyzed simultaneously. The research results show that for this coal mine, if there is carbon monoxide in the gas sample, the phenomenon of oxidation and temperature rising for coal exists in this mine; if there is C2H4 in the gas sample, the temperature of coal perhaps exceeds 130 °C. If the coal temperature is between 35 °C and 130 °C, prediction and forecast for coal spontaneous combustion depend on the value of Φ(CO)/K mainly; if the temperature of coal is between 130 °C and 300 °C, prediction and forecast for coal spontaneous combustion depend on the value of Φ(C2H6)/Φ(C2H2) and Φ(C2H6)/K. The research results provide experimental basis for the prediction of coal spontaneous combustion in Anyuan coal mine, and have better guidance on safe production of this coal mine.


