J. Cent. South Univ. Technol. (2010) 17: 388-393
DOI: 10.1007/s11771-010-0057-8 ![]()
Degradation of bond between steel bar and freeze-thaw concrete after electrochemical chloride extraction
GUO Yu-xia(郭育霞), GONG Jin-xin(贡金鑫)
State Key Laboratory of Coastal and Offshore Engineering, Dalian University of Technology, Dalian 116024, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract:
The effect of electrochemical chloride extraction (ECE) on bond strength between steel bar and freeze-thaw concrete contaminated by chloride was experimentally investigated for beam specimens with dimensions of 100 mm×100 mm×400 mm. During the experiment, 3% NaCl (vs mass of cement, mass fraction) was mixed into concrete to simulate chloride contamination, and the specimens experienced 0, 25, 50, 75 freeze-thaw cycles before ECE. In the process of ECE, different current densities and durations were adopted. It is indicated that the bond strength between reinforcement and concrete decreases with the increase of freeze-thaw cycles; the more the current and the electric quantity of ECE are, the more the loss of bond strength is; and the largest loss is up to 58.7%. So, it is important to choose proper parameters of ECE for the reinforced concrete structures contaminated by chloride and subjected to freeze-thaw cycles.
Key words:
concrete; bond strength; degradation; electrochemical chloride extraction; freeze-thaw cycles;
1 Introduction
Corrosion of steel bar in concrete due to chloride ions attacking is one of the major reasons that lead to the degradation of reinforced concrete (RC) structures throughout the world [1]. In order to extend the service life of existing RC structures contaminated by chloride ions, a non-destructive repair technique, i.e., electrochemical chloride extraction (ECE) of reinforced concrete, was developed by Federal Highway Administration of America (FHWA) and Strategic Highway Research Program (SHRP) in 1970’s, which involved mounting a temporary anode on the surface of the concrete and applying a direct current potential between this anode and the embedded steel bar to drive chloride ions away from the reinforcement and out of the concrete [2-3].
When current is impressed between steel bar embedded in concrete and external anode, cations and anions will migrate towards cathode (steel bar) and anode, respectively, and microstructure of concrete may be changed. Consequently, some side effects may occur, such as bond strength reduction, micro cracking, and alkali silica reaction (ASR) [4]. RASHEEDUZZAFAR et al [5] investigated the bond stress of cantilever beam specimens contaminated by chloride ions. It was found that the bond loss was up to 33% when the current density of 538 mA/m2 of steel area was adopted for 420 d. Commonly, current density used in ECE is higher than 538 mA/m2, so the bond loss may be more severe. IHEKWABA et al [6] reported that the pull-out bond degradation of steel bar in ECE concrete was 58% at a current density of 3.0 A/m2. Based on the test, HOSSEINI and KHALOO [7-8] concluded that ECE reduced the bond strength between steel bar and concrete after treatment, but the bond strength had a little recover after 56 d.
Most studies about the bond characteristics between reinforcement and concrete by ECE are based on the pull-out test, which is not in accordance with the stress of existent structures or structural members. On the other hand, in the progress of ECE, electric field acts between steel bar and anode of concrete surface, but in fact it only exists between one side and anode. Moreover, for the reinforced concrete structures or members located in cold region, deicing salt is one of chloride resources. If these structures or members are treated by ECE, they will experience some freeze-thaw cycles before treatment. Based on these, the bond behavior of beam specimens contaminated by chloride ions was studied after ECE and specimens experienced different freeze-thaw cycles before ECE.
2 Experimental
2.1 Materials
The materials used were grade 42.5 ordinary Portland cement, river sand with fineness modulus of 2.87, crushed lime stones with particle size of 5-20 mm, tap water, deformed steel bar with nominal diameter of 12 mm, yield strength of 399 MPa and ultimate strength of 595 MPa, and sodium chloride (NaCl). The mixing proportion of concrete is shown in Table 1. To simulate chloride contamination, 3% (mass fraction) NaCl (corresponding to 1.82% chloride ions, vs mass of cement) was added for contaminated specimens.
2.2 Methods
Beam specimen recommended by RILEM-FIP-CEB [9] was used in the test. The embedment length was 5 times as long as the diameter of steel bar, i.e., 60 mm, and PVC tube was adopted to separate steel bar from concrete. Specimens were demoulded after 24 h of moist curing in moulds and stored in standard maintaining house for 28 d. According to testing regulation,the specimens were kept in water for 2 d before freeze-thaw cycles. And it is necessary to show that PVC pipe covered on the free end of test bar was stuffed with wax to prevent steel bar from water. When the designed cycles (0, 25, 50, 75) of freeze-thaw were completed, the specimens were prepared for ECE.
To prevent the interaction of electric fields, every specimen was placed alone in a plastic container, and two blocks of each group were connected in series, as shown in Fig.1. The electrolyte was saturated with calcium hydroxide, the anode was stainless steel plate and the cathode was steel bar. In the process of ECE treatment, different current densities and electric quantity parameters were adopted, which were 1, 2, 3 A/m2 of steel area and 0.5, 1.5, 3.0 kA?h/m2, respectively. The current density was monitored and adjusted everyday to keep constant, the pH value of electrolyte was measured and the electrolyte was replaced if the pH was lower.
The test was carried out on hydraulic servo test equipment. Four-point load mode was adopted and the load was applied at a rate of around 50 μm/min of the free end of the rebar, and the bars’ slippages were measured by linear variable displacement transducers.s
Table 1 Mixing proportion of concrete
![]()
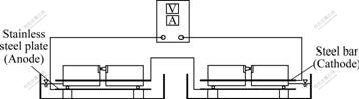
Fig.1 Schematic diagram of ECE
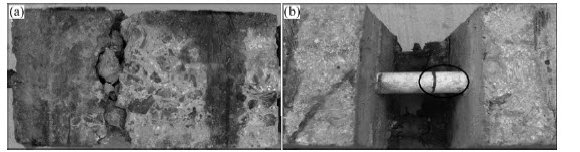
Fig.2 Bond failure modes(a) Flexural failure:(b)Pure slip failure
3 Results and discussion
3.1 Failure mode
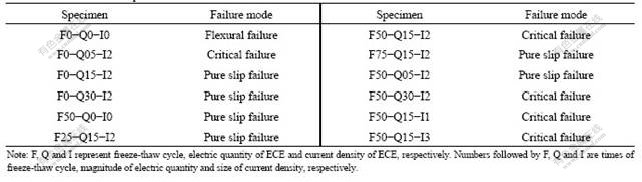
Three kinds of failure mode, i.e. flexural failure, pure slip failure and critical failure were observed and shown in Tab.2. For the flexural failure, during the progress of loading, obvious relative slippage between concrete and steel bar was not found, and concrete cracked (transverse crack) at the peak load. This failure was found in non-chloride control specimens. For those specimens, the bond between steel bar and concrete was perfect, and relative slip didn’t occur until the specimens failure .
For the pure slip failure, as shown in Fig.3, with the increase of load, relative slippage occurred between steel bar and concrete and the bar was slowly pulled out at the initial stage. When the peak load was obtained, the relative slippage sharply increased, and then the load slowly declined. Crack didn’t occur in the whole process. This failure was found in the specimens experienced some freeze-thaw cycles and ECE treatment. For these specimens, ECE treatment resulted in the degradation of bond behavior, as well as freeze-thaw cycle which damaged concrete and decreased bearing capacity, so the relative slip occurred under a lower load.
For the critical failure, similar to pure slip failure, with the increase of load, relative slippage occurred between steel bar and concrete and the bar was slowly pulled out at the early stage of loading. Crack was found in tensile region at peak load and didn’t develop when the load declined. This is a mode between the former two.
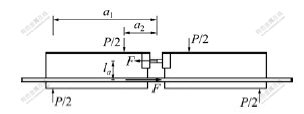
Fig.3 Schematic diagram of mechanical analysis of specimen
Table 2 Failure mode of specimens
3.2 Analysis of bond strength
The mechanical analysis of four-point bend beam specimens as shown in Fig.4 [10]。When loads of P/2 are symmetrically exerted on the two half beams of specimen,it can be derived that
![]() (1)
(1)
In which, ![]() is the distance between the centers of steel hinge and steel bar; a1and a2 are the distance from the center of steel hinge to the point of support reaction and to the point of loading, respectively.
is the distance between the centers of steel hinge and steel bar; a1and a2 are the distance from the center of steel hinge to the point of support reaction and to the point of loading, respectively.

Fig. 4 Bond strengh of specinens after treatment
If ![]() is defined the bond stress between concrete and steel bar under the total load of P, then
is defined the bond stress between concrete and steel bar under the total load of P, then
![]() (2)
(2)
Where, ![]() is the surface area of the embedded steel bar. In this study,
is the surface area of the embedded steel bar. In this study, ![]() and
and![]() were derived by Eq.(1) and Eq.(2) ,respectively, in which
were derived by Eq.(1) and Eq.(2) ,respectively, in which ![]() of Eq.(1) was the mean limit load of two specimens of each group. Tab.3 shows the results.
of Eq.(1) was the mean limit load of two specimens of each group. Tab.3 shows the results.
Table 3 Bond strength of specimens
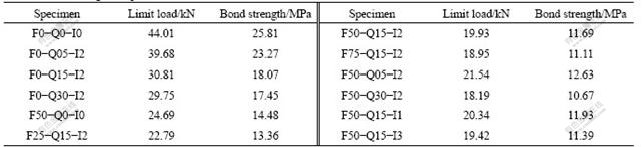
3.3 Effect of freeze-thaw cycles on bond strength
Fig.4 shows the bond strength of unfreeze-thawed specimens and freeze-thawed specimens after ECE treatment with a current density of 2 A/m2. It can be seen that the bond strengths of both freeze-thawed specimens and unfreeze-thawed specimens follow a similar decline trend with the increase of electric quantity and the bond strength of the former is lower than the latter after experienced ECE treatment of uniform current density and duration, which means that ECE reduce the bond strength of specimens and the bond degradation degree of the former is severer than that of the latter because of the effect of freeze- thaw. For unfreeze-thawed specimens , the bond strength decreases by 9.8%, 30% and 32.4% when the electric quantity increases from 0 to 500, 1500, 3000 A.h/m2, respectively. These of specimens experienced 50 freeze-thawed are 12.8%, 19.3% and 26.3%, respectively. Also, it can be observed that ECE has a greater influence on the bond strength of unfreeze-thawed specimenswithout . Due to the combined action of ECE and freeze-thaw, the loss of bond strength is up to 58.7% compared F50-Q30-I2 with F0-Q0-I0.
Fig.5 indicates that bond strength decreases with the increase of freeze-thaw cycles for specimens experiencing ECE treatment with the same current density of 2A/m2 and electric quantity of 1500 A.h/m2. Compared with unfreeze-thawed specimens, the ultimate bond strength of 25 cycle specimens dramatically reduces and after 25 cycles, its decrease rate turns slower.

Fig.5 Effect of freeze-thaw cycles on bond strength

Fig. 6 Effect of freeze-thaw cycles on the bond strength
3.4 Effect of ECE treatment on bond strength
Fig.6 shows the relationship between bond strength and electric quantity for specimens which experienced 50 freeze-thaw cycles and ECE treatment of 2A/m2 currentdensity. It is found that bond strength rapidly decreases at the beginning of treatment, after treatment of 500 A.h/m2, the decrease rate of bond strength declines. From 500 A.h/m2 to 3000 A.h/m2, the electric quantity and bond strength maintain an approximate linear relationship. It may be illuminated that ECE was efficient at the early stage, chloride ions are expelled from concrete and a lot of cations (K+、Na+ etc.) releasing from cement hydrates as well as from the pore solution and the electrolyte accumulate in the vicinity of steel bar and soften the concrete of interface. The result is in accordance with J.J. changs’[11] .Based on microhardness experiments, J.J. CHANG found that the reduction in microhardness occurs primarily in the first 14 d and after 2 weeks, the hardness is not greatly changed.Based on the control specimens of F50-Q0-I0 and F0-Q0-I0, the decrease rates of bond strength are are0, 12.8%, 19.3%, 26.3% and 43.9%, 51.1%, 54.7%, 58.7%, respectively.
Fig.7 shows the effect of current density on the bond strength. For specimens experienced 50 freeze-thaw cycle, the bond strength decreases from 14.48Mpa of control specimens to 11.93Mpa, 11.69Mpa and 11.39Mpa after ECE treatment of 1500 A·h/m2 and the current density is 1,2 ,3 A/m2 ,respectively. It means that impressed current would decrease the bond strength, but the degree of bond degradation is determined by electric quantity rather than current density magnitude. To some extent, the process of ECEof bond strength is not much changed.
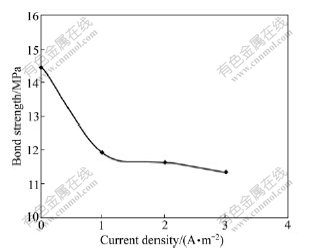
Fig. 7 Effect of current density on bond strength
3.5 Bond degradation mechanism
Bond strength of steel in concrete is developed by adhesion between the cement gel of concrete and the surface of steel bar, mechanical interlocking between deformed steel bar and concrete and friction generated on the interface of concrete and steel bar[12]. Any change of those three factors would lead to the decrease of bond strength. The degradation of bond in this test is the result of both freeze -thaw cycle and ECE.
According to the existing theory about freeze-thaw damage, concrete'sfreeze-thaw damage of is a low-cycle fatigue damage, and the volume expansion in freezing and shrinkage in thawing of pore solution correspond to the loading and unloading acting on the concrete micro-unit, respectively. The number of freezing and thawing is the number of fatigue cycle. Under those cyclic loading, the inherent microcrack or flaw of concrete is developed and fresh microcrack or flaw is formed, which result in the performance deterioration of concrete, such as compressive strength, dynamic elastic modulus. One of the major factors which influence the bond behavior between steel bar and concrete is concrete strength, the higher the compressive strength, the higher the chemical adhesion is. Therefore the decrease of compressive strength of concrete caused by freeze-thaw cycle leads to the decline of bond strength. On the other hand, in the progress of freeze thaw damage, the interior structure of the concrete turns looseen under the hydraulic pressure, which caused by the water in the capillary pores of cement paste upon freezing[13]. This interior change may weaken the friction generated on the interface of concrete and steel bar, consequently, the bond strength between concrete and steel bars is weakened.
In the process of ECE, the following factors may result in the bond degradation : (1)softening of interfacial concrete ECE treatment is characterized by a generation of hydroxyl ions (OH-) at the cathodic steel surface, and a concentration of sodium and potassium ions (Na+ and K+) from the hydrated cement in regions near the reinforcement due to the action of impressed current. A high concomitant concentration of OH-, Na+and K+ cations results in the formation sodium and potassium hydroxides with electrons being supplied by the rectifier. Theses alkali hydroxides are known to attack calcium silicates, thereby softening the concrete at the steel –concrete interface, resulting in a degradation of bond. NZERIBE M. Ihekwab[14] researched the mechanical properties of anodic and cathodic regions of ECE treated concrete and found that the compressive strength of anode was greater than that of cathode. J.J.CHANG [11] and G. BIKULCHUS[15] obtained the same conclusion by micro-hardness experiments about the concrete near the steel bar. This is a dominant factor. (2)changes of microstructure and compounds J.M.MIRANDA et al [16]think that ECE give rise to the reduction of some ferric compounds in the corrosion products to magnetite, increase the porosity of the mortar and weaken the bond between the steel bar and concrete. D.A.KOLEVAet al [17] think that tiny products formed prior to treatment diffuse through the material structure and fill in voids and open space,consequently, microstructure of concrete was changed, which influence the bond strength. (3)dissolution of corrosion product A. HOSSEINI et al[7] concluded that the contact area between concrete and bar was decreased because of the dissolution of corrosion product, which reduced the friction between concrete and steel bar. In this study, there is no corrosion product on the surface of steel bars, so this factor is not account for the bond degradation.
4 Conclusions
1) The combined action of freezing-thawing cycles and ECE treatment results in the degradation of bond between steel bar and concrete, and the degree of bond degradation is not a simple action effect superposition of the two factors. With the increase of current density, electric quantity of ECE and freeze-thaw cycles, the bond strength observably decreases. In this study, the most bond loss is up to58.7%.
2) The effect of ECE on the bond strength of unfreeze-thawed specimens is greater than that of freeze-thawed specimens.
3) For freeze-thawed specimens, the effect of magnitude of current density is unconspicuous and in some conditions, the duration of ECE treatment could be shorted by adopt a high current density.
References
[1] SWAMY R N, STEPHEN MCHUGH. Effectiveness and structural implications of electrochemical chloride extraction from reinforced concrete beams [J].Cement & Concrete Composites, 2006, 28(8): 722-733.
[2] BUENFELD N R, BROOMFIELD J P. Influence of electrochemical chloride extraction on the bond between steel and concrete [J]. Magazine of Concrete Research, 2000, 52(2): 79-91.
[3] TOUMI A, FRANCOIS R, ALVARADO O, Experimental and numerical study of electrochemical chloride removal from brick and concrete specimens[J].Cement and Concrete Research, 2007,37(1):54-62
[4] ORELLAN J C, ESCADEILLAS G. Electrochemical chloride extraction: efficiency and side effects[J] , Cement and Concrete Research, 2004, 34(2): 227-234.
[5] RASHEEDUZZAFAR M G. Degradation of bond between reinforcing steel and concrete due to cathodic protection current[J], ACI Materials Journal, 1993,90( 1): 17-25.
[6] IHEKWABA N M, HOPE BB, HANSSON C M. Pull-out and bond degradation of steel rebars in ECE concrete[J], Cement and Concrete Research, 1996, 26(2): 267-282.
[7] HOSSEINI A, KHALOO ALI R. Study of electrochemical chloride extraction as a non-destructive repair method: part Ⅰ.discrete test samples [J], Asian journal of civil engineering :building and housing, 2005, 6 (3): 167-182.
[8] HOSSEINI A, KHALOO A R. Study of electrochemical chloride extraction as a non-destructive repair method: part Ⅱ. structural element test sample [J], Asian journal of civil engineering : building and housing, 2005, 6 (4): 247-255.
[9] WANG Chuan-zhi, TENG Zhi-ming. Theory of reinforced concrete structure[M]. Beijing: Architecture Industry Press, 1985. (in Chinese)
[10] GONG Jin-xing, HE Shi-qin, GUO Yu-xia, Influence of freezing and thawing cycles on bond characteristics of steel bar and concrete in salt environment[J]. Journal of Dalian University of Technology. 2005, 45(3): 405-409. (in chinese )
[11] CHANG J J. Bond degradation due to the desalination process[J], Construction and Building Materials, 2003, 17(4): 281-287.
[12] JI Xiao-dong, SONG Yu-pu, LIU Yuan. Effect of freeze-thaw cycle on bond strength between steel bars and concrete [J]. Journal of Wuhan University of Technology—Materials Science Edition. 2008,23(4): 584-588.
[13] RACGEK H DETWILER, BRIAN J DALGLEISH. Assessing the durability of concrete in freezing and thawing [J].ACI Mater.J., 1989, 86(1):29-35.
[14] NZERIBE M. IHEKWABA & BRIAN B. HOPE, Mechanical properties of anodic and cathodic regions of ECE treated concrete[J], Cement and Concrete Research, 1996,26(5): 771-780.
[15] BIKULCHUS, Chloride removal from reinforced concrete and relevant loss of strength, Protection of Metals 2005, 41(5) : 484–486.
[16] MIRANDA J M, COBO A, OTERO E, GONA?LEZ, Limitations and advantages of electrochemical chloride removal in corroded reinforced concrete structures[J]. Cement and Concrete Research, 2007,37(4): 596-603.
[17] KOLEVA D A, BREUGEL K, WIT J H W. Correlation of microstructure, electrical properties and electrochemical phenomena in reinforced nortar. Part I: Microstructural observations and electrical properties[J].Materials characterization, 2008, 59(3): 290-300.
Foundation item: Project(IRT0518) supported by the Program of Innovative Team of the Ministry of Education of China
Received date: 2009-05-30; Accepted date: 2009-09-26
Corresponding author: GONG Jin-xin, Professor; Tel: +86-13889608239; E-mail: gong_jx.vip@eyou.com
(Edited by CHEN Wei-ping)
Abstract: The effect of electrochemical chloride extraction (ECE) on bond strength between steel bar and freeze-thaw concrete contaminated by chloride was experimentally investigated for beam specimens with dimensions of 100 mm×100 mm×400 mm. During the experiment, 3% NaCl (vs mass of cement, mass fraction) was mixed into concrete to simulate chloride contamination, and the specimens experienced 0, 25, 50, 75 freeze-thaw cycles before ECE. In the process of ECE, different current densities and durations were adopted. It is indicated that the bond strength between reinforcement and concrete decreases with the increase of freeze-thaw cycles; the more the current and the electric quantity of ECE are, the more the loss of bond strength is; and the largest loss is up to 58.7%. So, it is important to choose proper parameters of ECE for the reinforced concrete structures contaminated by chloride and subjected to freeze-thaw cycles.


