J. Cent. South Univ. (2017) 24: 2793-2798
DOI: https://doi.org/10.1007/s11771-017-3693-4
A new approach for deposition of silver film from AgCl through successive ionic layer adsorption and reaction technique
Johnson Henry1, Arockiasamy Ajaypraveenkumar1, Ganesan Sivakumar2, Kannusamy Mohanraj1
1. Department of Physics, Manonmaniam Sundaranar University, Tirunelveli-627 012, Tamil Nadu, India;
2. CISL, Department of Physics, Annamalai University, Annamalai nagar-608 002, Tamil Nadu, India
 Central South University Press and Springer-Verlag GmbH Germany,part of Springer Nature 2017
Central South University Press and Springer-Verlag GmbH Germany,part of Springer Nature 2017
Abstract:
An attempt was made to deposit thin film of silver onto the glass substrate by using AgCl precursor, instead of conventional precursor AgNO3 with vitamin C by inexpensive and convenient successive ionic layer adsorption and reaction (SILAR) method. The deposited silver thin film was characterized by X-ray diffraction (XRD) analysis, scanning electron microscope (SEM), UV-visible and electrical I-V study. The diffraction study showed FCC structure of metallic silver in good agreement with the standard values of JCPDS (04–0783). SEM reveals flower like nano particles produced on the substrate. The surface plasmon resonance (SPR) peak in the UV-visible spectrum shows maximum absorption at 350 nm. The film shows an ohmic behavior and its electrical resistivity was found ~103 Ω·cm at room temperature.
Key words:
thin films; metals; materials characterization; sol-gel; optical properties; silver;
1 Introduction
In recent years noble metal nanoparticles have been the subjects of focused researchers due to their unique electronic, optical, mechanical, magnetic and chemical properties which are significantly different from those of bulk materials [1, 2]. It could be attributed to their small sizes and large specific surface area. The metallic nanoparticles have found uses in many applications in different fields as catalysis [3], electronics [4] medicine [5] and photonics [6]. Nowadays, several types of metal nanoparticles such as gold, silver, zinc and copper have been successfully synthesized for various anticipated applications that include medicine and diagnosis, nutrition and neutraceuticals and optoelectronics [7–11]. Among them, silver nanoparticles (AgNPs) have attracted special attention because of their widespread physical and chemical nature and tunable surface plasmon resonance [12]. A variety of methods have reported for synthesis of silver nanoparticles such as precipitation [8], spray pyrolysis [13], hydrothermal [14], solvothermal [15] and sonochemical method [16] with the assistance of sodium borohydride [8], formalin [17], sodium dodecyl sulphate, hydrazine hydrate [18] and chloroform [19]. However, the synthesized NPs could not be used in the medical application owing to these reagents being toxic, and harmful to human health.
To overcome this problem, green/bio synthesis method is encouraged in the recent years, since the route is safe, eco-friendly and inexpensive. Subsequently, a variety of biomolecules, such as pullulan, citrates, glucose, vitamin C (ascorbic acid) and acetic acid [20–24], living organisms like microbes [25], fungi [26] and parts of plants [27], have been used for the synthesis of AgNPs from the conventional precursor AgNO3. Although, the AgCl by-product will be formed for the synthesis of AgNPs, owing to some amount of chloride (AgCl) content present in the plants [2, 27]. Hence, the formation of the by-product is inevitable and obviously visible that a high cost metal is being wasted. To know to our knowledge, there is no work available for the deposition of silver thin film using precursor AgCl with bio-reducer vitamin C. Therefore, we approach a simple route for the extraction of Ag from the precursor AgCl, instead of conventional AgNO3.
In this preparation process we have adopted the successive ionic layer adsorption and reaction (SILAR) method which is also called as modified chemical bath deposition. The name SILAR was ascribed by NICOLAU in the mid-1980s [28]. The SILAR method was first reported by RISTOV et al [29] (1985) for Cu2O thin film. SILAR has a number of advantages: 1) it offers an extremely easy way to dope films; 2) it does not require high quality target and vacuum at any stage; 3) the deposition rate and the thickness of the film can be easily controlled; 4) operating at room temperature can produce films on less robust materials; 5) it does not cause local overheating, which can be detrimental for materials to be deposited; 6) there are virtually no restrictions on the substrate material, dimensions or its surface profile; 7) it is simple, less expensive and less time consuming and 8) it is suitable for deposition of large area thin films. The detailed explanation of the SILAR method is given by PATHAN and LOKHANDE (2004) [30]
2 Experimental details
A. R. grade of AgCl (99%), vitamin C (ascorbic acid) (99%) and NH3 (25%) were used without any modification. Substrate cleaning played an important role in the deposition of thin films and the procedure was adopted according to the earlier report [31]. The experimental process consisted of four beakers; the first beaker contained the precursor solution (40 mL) of 0.1 mol/L AgCl dissolved in NH3; the second and fourth beakers had sufficient amount of distilled water to remove loosely adsorbed cations from the substrate; the third beaker contained 0.1 mol/L reducing agent vitamin C. In SILAR method, the growth kinetics of a thin film deposition process involves ion-by-ion deposition at nucleation sites on the immersed surfaces. In the typical deposition, substrates were immersed separately in precursor and vitamin C solution with simultaneous rinsing with distilled water between every immersion to avoid any precipitation. First, the ultrasonically cleaned substrate was immersed vertically into the cationic precursor solution for 30 s in which silver amine complex was adsorbed on the glass substrate. Consequently, the substrate was rinsed with distilled water for 10 s to remove the loosely bonded ions, again immersed in the anionic precursor solution for 30 s. Instantly, the [Ag(NH3)2]+ complex reduced as Ag+ ions on a glass substrate and finally rinsed with distilled water for 10 s to remove the unreacted vitamin C, Ag ions and loosely bonded powdery Ag particles. The above four steps (Fig. 1) were one SILAR cycle. Similarly, 30 SILAR cycles were made to deposit silver thin film.
The film thickness was determined by using the standard procedure as reported in Ref. [32] and it is found to be 1163 nm. Thickness of the present result is low (1163 nm) in comparison to the earlier reports on silver thin film (1429 nm, 1780 nm and 2480 nm) deposited by green synthesis method [2, 27]. The structural characteristics of the film were analyzed by X-ray diffraction (XRD) pattern recorded using PANalytical X`Pert Pro powder diffractometer (λ= 1.5406 ) in the range of 2θ=10°–80°. The surface morphology of the film was recorded by JEOL scanning electron microscope (SEM, model JEM-5610 LV). The optical absorption spectrum was recorded using JASCO UVIDEC-650 UV-Vis spectrophotometer and electrical property of the film was measured with AUT85670 set up.
) in the range of 2θ=10°–80°. The surface morphology of the film was recorded by JEOL scanning electron microscope (SEM, model JEM-5610 LV). The optical absorption spectrum was recorded using JASCO UVIDEC-650 UV-Vis spectrophotometer and electrical property of the film was measured with AUT85670 set up.
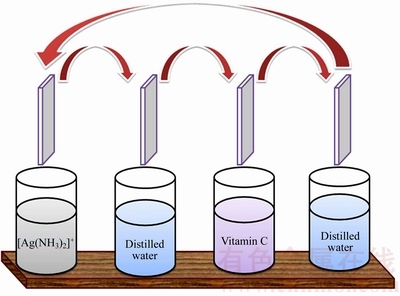
Fig. 1 Schematic representation of silver thin film deposited by SILAR method
3 Results and discussions
Figure 2 shows the XRD pattern of silver thin film that indicates three distinctive reflections at (111), (200) and (311) corresponding to the FCC structure of metallic silver. The observed d-spacing value well coincide with the standard data [JCPDS Card No.: 04–0783] and an important notice that there is no impurity peak in the diffraction pattern in comparison to the earlier reports [2, 27, 33]. The observation implies that vitamin C had completely reduced the AgCl and pure silver particles were produced. Among the FCC planes, plane (111) is more intense, which may be been due to the predominant orientation of the (111) plane. This indicates that they are not isotropically oriented in a crystallographic sense, probably because they are capped by distinct crystallographic faces. No AgCl peak is present in the XRD, which indicates that the sample consists of pure FCC silver only [34]. The obtained XRD intensity is higher than the earlier report of Ag thin films prepared by DC magnetron sputtering [35]. The high intensity and sharp peaks in XRD patterns confirm the highly oriented nature of the Ag films prepared in this study.
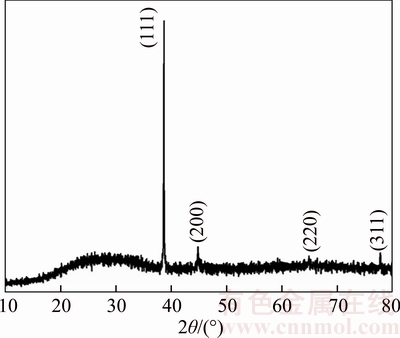
Fig. 2 XRD pattern of silver thin film
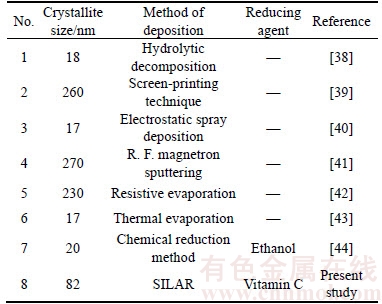
The crystallite size was estimated by using the Scherrer’s formula D=0.9λ/βcosθ [36] and the dislocation density was calculated from the formula δ=1/D2 [37], where D is the crystallite size, λ is the wavelength of XRD source, β is full width at half maximum, θ is Bragg angle and δ is the dislocation density. The calculated crystallite size and dislocation density is found to be 82.5 nm and 1.47×1014 line/m, respectively. From Table 1 it is clearly seen that the obtained crystallite size is lower than that of silver films prepared by thermal methods.
Table 1 Comparison of crystallite size of silver thin films with other deposition methods
When AgCl is dissolved in ammonia solution, silver amine complex will be formed while the reducing agent vitamin C produces electrons by oxidation reactions [10, 21] (equations (1, 2)). Subsequently, these electrons reduce the silver amine complex to Ag0 in the solution (Eq. (3)).
AgCl+2NH3→[Ag (NH3)2] ++Cl– (1)
C6H8O6 C6H6O6+2H++2e– (2)
C6H6O6+2H++2e– (2)
[Ag(NH3)2]++e–→Ag+2NH3 (3)
Thin film deposition proceeds four phases, namely nucleation, aggregation, coalescence and growth phase.During the nucleation phase, the silver ions come closer to each other and form cationic nuclei on the substrate surface. In alternative steps, the substrate is dipped into the vitamin C solution, which reduces the silver ions to silver nanoparticles (Eq. (3)). This process is called nucleation. These nucleation centers act as a foundation for aggregation, because aggregation occurs on the substrate surface once a few particles are attracted to the substrate. This process is called aggregation. In next step many silver nanoparticles are merged with each other. This process is called coalescence. Finally the thin films grow with certain thickness on the substrate by stacking of the silver nanoparticles. This phase is called growth phase.
Figure 3 shows the SEM image of the silver thin film in which a number of non-spherical silver particles are distributed uniformly and seems to be the densest surface. In the high magnification image (Fig. 3(b)), a number of distinct 3D flowers like AgNPs are clearly seem. The average diameter of the particle is in the range of 1–5 μm.
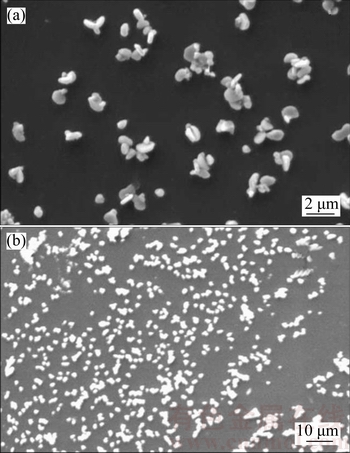
Fig. 3 SEM image of silver thin films with different magnifications
Since the average crystallite size obtained by the XRD is smaller than that of SEM image, the observed difference can be attributed to the fact that XRD measurements consider crystallite sizes as sizes of “coherently diffracting domains” of crystals while grains may contain several of these domains. Another reason for these differences could be a possible plasmon interaction of SEM electron bundle with Ag-nano particle surface, which appears in magnification size effect [33]. Similar morphology was obtained in earlier report of Ag thin film prepared by DC magnetron sputtering [35].
It can be seen from the UV-Visible spectrum (Fig. 4) that the maximum absorption observed around 350 nm attributed to the surface plasmon resonance (SPR) of silver particles. The result is in good agreement with the earlier work on silver thin films reported by ZHANG et al [45] and LUU et al [46]. The absorption tail observed at 500–600 nm may be attributed to the formation of nanoflower structures of silver [47] obviously visible in the SEM image (Fig. 3).
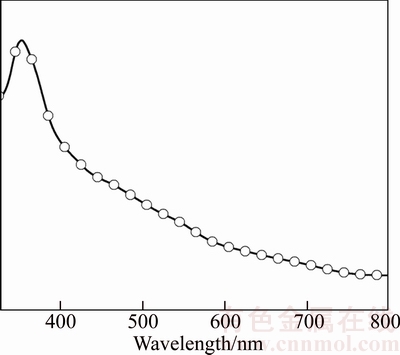
Fig. 4 UV-visible absorption spectrum of silver thin film
Figure 5 shows the I-V characteristics of silver thin film that shows ohmic and linear behavior in the I–V curve. The resistivity of the deposited film was calculated by using the formula given below [48]
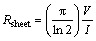 (4)
(4)
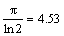 (5)
(5)
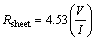 (6)
(6)
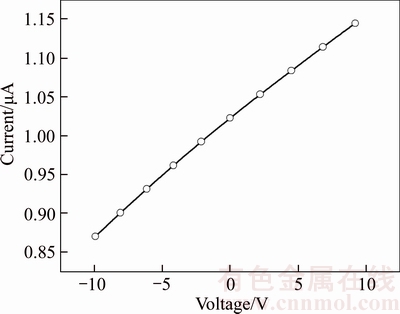
Fig. 5 I–V characteristics of silver thin film
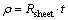 (7)
(7)
The resistivity of the film was found about 36 kΩ·cm and is in good agreement with the earlier report on silver thin films by FENG and YAN [49].
4 Conclusions
Thin film of silver was deposited onto the glass substrate from AgCl with the assistance of biomolecule vitamin C by inexpensive and convenient SILAR method. The XRD pattern of the thin film confirms the formation of the FCC structure of the silver thin film as witnessed by the strong SPR band in the UV-visible spectrum and absorption tail observed at 500–600 nm attributed to the formation of flower like silver particles. The electrical resistivity value of the film is found ~103 Ω·cm at room temperature. It is concluded from the experimental studies that the precursor AgCl can be used for the deposition of nanoflower like Ag film with the assistance of vitamin C by the simple SILAR method.
Acknowledgements
The authors are thankful to the DST-FIST and UGC-SAP, New Delhi for providing the financial support to the Department of Physics, Manonmaniam Sundaranar University and the authors are thankful to the Prof. and Head, Department of Physics, Annamalai University for recording SEM images.
References
[1] VALKONEN E, RIBBING C G, SUNDGREN J E. Optical constants of thin silver and titanium nitride films [J]. Proc of SPIE, 1986, 652: 235–242.
[2] SHINDE N M, LOKHANDE A C, LOKHANDE C D. A green synthesis method for large area silver thin film containing nanoparticles [J]. J Photochem Photobiol B, 2014, 136: 19–25.
[3] VANAJA M, PAULKUMAR K, BABURAJA M, RAJESHKUMAR S, GNANAJOBITHA G, MALARKODI C, SIVAKAVINESAN M, ANNADURAI G. Degradation of methylene blue using biologically synthesized silver nanoparticles [J]. Bioinorganic Chem Appl, 2014, 2014: 742346.
[4] CHEN D, QIAO X, QIU X, CHEN J. Synthesis and electrical properties of uniform silver nanoparticles for electronic applications [J]. J Mater Sci, 2014, 44: 1076–1081.
[5] AZLIN-HASIM S, CRUZ-ROMERO M C, GHOSHAL T, MORRIS M A, CUMMINS E, KERRY J P. Application of silver nanodots for potential use in antimicrobial packaging applications [J]. Innov Food Sci & Emerg Technol, 2015, 27: 136–143.
[6] HUANG S Y, PENG C C, TU L W, KUO C T. Enhancement of luminescence of nematic liquid crystals doped with silver nanoparticles [J]. Mol Cryst Liq Cryst, 2015, 507: 301–306.
[7] ZHOU J, RALSTON J, SEDEV R, BEATTIE D A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability [J]. J Colloid Interface Sci, 2015, 331: 251–262.
[8] HE S, CHEN H, GUO Z, WANG B, TANG C, FENG Y. High- concentration silver colloid stabilized by a cationic Gemini surfactant [J]. Colloids Surf A, 2015, 429: 98–105.
[9] KULKARNI N, MUDDAPUR U. Biosynthesis of metal nanoparticles: A review [J]. Journal of Nanotechnology, 2014, 2014: 510246.
[10] DEMIRKIRAN N. A study on preparation of copper powder without an external electrical current source [J]. Rev Chim, 2015, 64: 378–381.
[11] HUANG X, EL-SAYED M A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy [J]. J Adv Res, 2010, 1: 13–28.
[12] WANG J F, LI H J, ZHOU Z Y, LI X Y, LIU J, YANG H Y. Tunable surface-plasmon-resonance wavelength of silver island flms [J]. Chin Phys B, 2010, 19: 11731.
[13] PINGALI KC, ROCKSTRAW D A, DENG S. Silver nanoparticles from ultrasonic spray pyrolysis of aqueous silver nitrate [J]. Aerosol Sci Technol, 2005, 39: 1010–1014.
[14] ZOU J, XU Y, HOU B, WU D, SUN Y. Controlled growth of silver nanoparticles in a hydrothermal process [J]. China Particuology, 2007, 5: 206–212.
[15] ROSEMARY M J, PRADEEP T. Solvothermal synthesis of silver nanoparticles from thiolates [J]. J Colloid Interface Sci, 2007, 268: 81–84.
[16] SHIN U S, HONG H K, KIM H W, GONG M S. Preparation of silver nanoparticles in ultrasonic vibration-induced nanodroplets of isopropyl alcohol in combination with ionic liquids [J]. Bull Korean Chem Soc, 2011, 32: 1583–1586.
[17] SZCZEPANOWICZ K, STEFA SKA J, SOCHA R P, WARSZY
SKA J, SOCHA R P, WARSZY SKI P. Preparation of silver nanoparticles via chemical reduction and their antimicrobial activity [J]. Physicochem Probl Miner Process, 2010, 45: 85–98.
SKI P. Preparation of silver nanoparticles via chemical reduction and their antimicrobial activity [J]. Physicochem Probl Miner Process, 2010, 45: 85–98.
[18] GUZM N M G, DILLE J, GODET S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity [J]. Int J Chem Biomolecular Eng, 2009, 2: 104–111.
N M G, DILLE J, GODET S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity [J]. Int J Chem Biomolecular Eng, 2009, 2: 104–111.
[19] VODNIK V V,  Z V, NEDELJKOVI
Z V, NEDELJKOVI J M. Optical properties of shaped silver nanoparticles [J]. J Nanosci Nanotechnol, 2009, 8: 1–5.
J M. Optical properties of shaped silver nanoparticles [J]. J Nanosci Nanotechnol, 2009, 8: 1–5.
[20] KANMANI P, LIM S T. Synthesis and characterization of pullulan-mediated silver nanoparticles and its antimicrobial activities [J]. Carbohydr Polymers, 2013, 97: 421–428.
[21] GROUP L F, LONGO E, LEITE E R, CAMARGO E R. Moderationg effect of ammonia on particle growth and stability of quasi- monodisperse silver nanoparticles synthesized by Turkevich method [J]. J Colloid Interface Sci, 2011, 360: 355–358.
[22] STEVANOVI M, BRA
M, BRA KO I, MILENKOVI
KO I, MILENKOVI M, FILIPOVI
M, FILIPOVI N,NUNI
N,NUNI J, FILIPI
J, FILIPI M, USKOKOVI
M, USKOKOVI D P. Multifunctional PLGA particles containing poly(l-glutamic acid)-capped silver nanoparticles and ascorbic acid with simultaneous antioxidative and prolonged antimicrobial activity [J]. Acta Biomaterialia, 2014, 10: 151–162.
D P. Multifunctional PLGA particles containing poly(l-glutamic acid)-capped silver nanoparticles and ascorbic acid with simultaneous antioxidative and prolonged antimicrobial activity [J]. Acta Biomaterialia, 2014, 10: 151–162.
[23] QIN Y, JI X, JING J, LIU H, WU H, YANG W. Size control over spherical silver nanoparticles by ascorbic acid reduction [J]. Colloid Surf A, 2010, 372: 172–176.
[24] WU Song-ping, MENG Shu-yuan. Preparation of ultrafine silver powder using ascorbic acid as reducing agent and its application in MLCI [J]. Mater Chem Phys, 2005, 89: 423–427.
[25] BEHERA S S, JHA S, ARAKHA M, PANIGRAHI T K. Synthesis of silver nanoparticles from microbial source—A green synthesis approach, and evaluation of its antimicrobial activity against Escherichia Coli [J]. Int J Eng Res Appl, 2013, 3: 58–62.
[26] VIGNESHWARAN N, ASHTAPUTRE N M, VARADARAJAN P V, NACHANE R P, PARALIKAR K M, BALASUBRAMANYA R H. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus [J]. Mater Lett, 2007, 61: 1413–1418.
[27] SHINDE N M, LOKHANDE A C, BAGI J S, LOKHANDE C D. Biosynthesis of large area (30×30 cm2) silver thin films [J]. Mat Sci Semiconductor Process, 2014, 22: 28–36.
[28] NICOLAU Y F. Solution deposition of thin solid compound films by a successive ionic-layer adsorption and reaction process [J]. Applications of Surface Science, 1985, 22/23: 1061–1074.
[29] RISTOV M, SINADINOVSKI G J. Chemical deposition of Cu2O thin films [J]. Thin Solid Films, 1984, 123: 63–67.
[30] PATHAN H M, LOKHANDE C D. Deposition of metal chalcogenide thin films by successive ionic layer adsorption and reaction (SILAR) method [J]. Bull Mater Sci, 2004, 27: 85–111.
[31] SABLI N, TALIB Z A, YUNUS W M M, ZAINAL Z, HILAL H S, FUJI M. CuZnSnSe thin film electrodes prepared by vacuum evaporation: Enhancement of surface morpholog and photoelectrochemical characteristics by argon gas [J]. Materials Science Forum, 2015, 756: 273–280.
[32] RIVEROUS E, ROMERO E, GORIDLLO G. Synthesis and characterization of highly transparent conductive SnO2:F and In2O3:Sn thin films deposited by spray pyrolysis method [J]. Brazilian J Phys, 2006, 36: 1042–1045.
[33] KARINIEMI M, NIINISTO J, HATANPAA T, KEMELL M, SAJAVAARA T, RITALA M, LESKELA M. Plasma-enhanced atomic layer deposition of silver thin films [J]. Chem Mater, 2011, 23: 2901–2907.
[34] HELMLINGER J, PRYMAK O, LOZA K, GOCYLA M, HEGGEN M, EPPLE M. On the crystallography of silver nanoparticles with different shape [J]. Cryst Growth Des, 2016, 16: 3677–3687.
[35] HAJAKBARI F, ENSANDOUST M. Study of thermal annealing effect on the properties of silver thin films prepared by DC magnetron sputtering [J]. Acta Physica Polonica A, 2016, 129: 680–682.
[36] SUBRAMANIAM E P, RAJESH G, MUTHUKUMARASAMY N, THAMBIDURAI M, ASOKAN V, VELAUTHAPILLAI D. Solar cells of Cu2ZnSnS4 thin films prepared by chemical bath deposition method [J]. Indian J Pure & Appl Phys, 2014, 52: 620–624.
[37] ILICAN S, CAGLAR Y, CAGLAR M. Preparation and characterization of ZnO thin films deposited by sol-gel spin coating method [J]. J Optoelectron Adv Mater, 2008, 10: 2578–2583.
[38] DIMITRIJEVI R, CVETKOVI
R, CVETKOVI O, MIODRAGOVI
O, MIODRAGOVI Z, SIMI
Z, SIMI M, MANOJLOVI
M, MANOJLOVI C D, JOVI
C D, JOVI V. SEM/EDX and XRD characterization of silver nanocrystalline thin film prepared from organometallic solution precursor [J]. J Min Metall Sect B-Metall B, 2013, 49: 91–95.
V. SEM/EDX and XRD characterization of silver nanocrystalline thin film prepared from organometallic solution precursor [J]. J Min Metall Sect B-Metall B, 2013, 49: 91–95.
[39] ALI M K M, IBRAHIM K, MKAWI E M, PAKHURUDDIN M Z. Surface morphology and structural properties of silver thin films prepared on polyethylene terephthalate (PET) substrate by screen printing technique [J]. Advanced Materials Research, 2012, 364: 110–114.
[40] THIWAWONG T, ONLAOR K, TUNHOO B. A humidity sensor based on silver nanoparticles thin film prepared by electrostatic spray deposition process [J]. Advances in Materials Science and Engineering, 2013, 2013: 640428.
[41] KAPAKLIS V, POULOPOULOS P, KAROUTSOS V, MANOURAS T, POLITIS C. Growth of thin Ag films produced by radio frequency magnetron sputtering [J]. Thin Solid Films, 2006, 510: 138–142.
[42] ESFANDIAR A, SAVALONI H, PLACIDO F. On the fabrication and characterization of graded slanted chiral nano-sculptured silver thin films [J]. Physica E, 2013, 50: 88–96.
[43] CHINNASAMY R, KRISHNAMOORTHY R, SHAMUGAM R K, THANGAVELU R R. Synthesis and antibacterial studies of nanostructured Ag thin films [J]. Advanced Materials Research, 2013, 678: 291–296.
[44] CHOY K, KIM I H, SHIN K S. Green synthesis of Ag thin films on glass substrates and their application in surface-enhanced Raman scattering [J]. Bull Korean Chem Soc, 2013, 34: 2942–2946.
[45] ZHANG D, QI L, YANG J, MA J, CHENG H, HUANG L. Wet chemical synthesis of silver nanowire thin films at ambient temperature [J]. Chem Mater, 2004, 16: 872–876.
[46] LUU Q N, DOORN J M, BERRY M T, JIANG C, LIN C, MAY P S. Preparation and optical properties of silver nanowires and silver-nanowire thin films [J]. J Colloid Interface Sci, 2011, 356, 151–158.
[47] GIANNINI V, RODR GUEZ-OLIVEROS R,S
GUEZ-OLIVEROS R,S NCHEZ-GIL J A. Surface plasmon resonances of metallic nanostars/nanoflowers for surface-enhanced Raman scattering [J]. Plasmonics, 2010, 5: 99–104.
NCHEZ-GIL J A. Surface plasmon resonances of metallic nanostars/nanoflowers for surface-enhanced Raman scattering [J]. Plasmonics, 2010, 5: 99–104.
[48] OKEREKE N A, EKPUNOBI A J. Structural and electrical characteristics of silver selenide thin films [J]. J Optoelectronics and Biomedical Mater, 2011, 3: 51–55.
[49] FENG Z, YAN F. Preparation and resistivity study of silver nanocomposite films using spin-assisted layer-by-layer assembly [J]. Surf Interface Anal, 2008, 40: 1523–1528.
(Edited by YANG Hua)
Cite this article as:
Johnson Henry, Arockiasamy Ajaypraveenkumar, Ganesan Sivakumar, Kannusamy Mohanraj. A new approach for deposition of silver film from AgCl through successive ionic layer adsorption and reaction technique [J]. Journal of Central South University, 2017, 24(12): 2793–2798.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-017-3693-4Received date: 2016-07-01; Accepted date: 2016-12-27
Corresponding author: Kannusamy Mohanraj, PhD, Assistant Professor; Tel: +91–9788083079; E-mail: kmohanraj.msu@gmail.com; mohanraj@ msuniv.ac.in
Abstract: An attempt was made to deposit thin film of silver onto the glass substrate by using AgCl precursor, instead of conventional precursor AgNO3 with vitamin C by inexpensive and convenient successive ionic layer adsorption and reaction (SILAR) method. The deposited silver thin film was characterized by X-ray diffraction (XRD) analysis, scanning electron microscope (SEM), UV-visible and electrical I-V study. The diffraction study showed FCC structure of metallic silver in good agreement with the standard values of JCPDS (04–0783). SEM reveals flower like nano particles produced on the substrate. The surface plasmon resonance (SPR) peak in the UV-visible spectrum shows maximum absorption at 350 nm. The film shows an ohmic behavior and its electrical resistivity was found ~103 Ω·cm at room temperature.


