Article ID: 1003-6326(2005)03-0612-03
Deformation of Zr41Ti14Cu12.5Ni10Be22.5 bulk amorphous alloy under isobaric pressure in super-cooled liquid region
ZHANG Ke-qin(张克勤)1, LU Qi-zhu(卢启柱)2
(1. Key Laboratory of Metastable Materials Science and Technology, Yanshan University,
Qinhuangdao 066004, China;
2. College of Information Science and Engineering, Yanshan University,
Qinhuangdao 066004, China)
Abstract:
The curve of crystallization transition during continuous heating for the Zr41Ti14Cu12.5Ni10Be22.5 bulk amorphous alloy was measured by means of dilatation(Fully automatic transformation recording/measuring instrument) and X-ray diffraction(XRD) method. The deformation behavior of the alloy at various heating rates in the supercooled liquid region was studied. The results show that the glass transition temperature of the alloy increases slightly and the supercooled liquid region(SLR) increases significantly with increasing heating rate. The deformation amount under isobaric pressure of 1N for the alloy in the SLR increases with increasing heating rate. As the heating rate of the alloy increases from 5 to 100℃/min, the amount of deformation of the alloy increases from 8.3% to 45%.
Key words:
Zr41Ti14Cu12.5Ni10Be22.5 alloy; bulk amorphous alloy; supercooled liquid region; deformation CLC;
number: TG146 Document code: A
1 INTRODUCTION
The formation of metallic glass by melt quenching from the liquid state with high cooling rate(106K/s) was first proposed by Duwez et al[1] in 1960. Since then, a considerable number of metallic glass and nanostructured systems have been prepared by this technique[2-4] and some industrial applications are well established. However, the high cooling rate limits the sample geometry to thin sheets and lines, which are unlikely to be widely applied. During the past years, a number of complex multi-component bulk amorphous alloys with excellent glass forming ability(GFA) are developed[5-12]. The minimum critical cooling rate for glass formation has been reported to be about 1K/s[8]. These amorphous alloys, which exhibit advantageous properties such as high thermal stability and easy processing[8-17], are believed to have considerable potential as advanced engineering materials. In addition to their interesting applications, these alloys, with an excellent stability in a larger super-cooled liquid region (SLR), provide a large experimental time scale to measure their physical properties, such as specific heat[10], diffusion[11], and viscosity[12] in the super-cooled liquid state. The ZrTiCuNiBe alloy system represents one group of such alloys, which shows the best glass forming ability[8, 9]; and its GFA approaches that of the traditional oxide glasses. The ability to form amorphous alloys very easily in large cross sections from common metallic elements, and possessing unique properties have aroused great interest and led to rapid development.
Multi-component bulk amorphous alloys have been developed in recent years. The bulk amorphous alloy in Zr-Ti-Cu-Ni-Be system is an alloy system with the large glass forming ability(GFA). The amorphous alloy has drawn plenty of intensive investigations because of its unique mechanical and physical properties[18, 19]. One of the most important characteristics of the Zr-Ti-Cu-Ni-Be system bulk amorphous alloy is the wide SLR(ΔT ). ΔT is defined as the difference between the glass transition temperature(Tg) and crystallization temperature(Tx). When ΔT(=Tx-Tg) reaches 100K, it can provide conditions for studying the mechanical behavior of bulk amorphous alloy in the SLR. In the present paper the deformation behavior of Zr41Ti14Cu12.5Ni10Be22.5 alloy under isobaric pressure at various heating rates in the supercooled liquid region is studied.
2 EXPERIMENTAL
The bulk amorphous alloy with composition of Zr41Ti14Cu12.5Ni10Be22.5 was quenched in water to get rods(diameter of 8mm).The amorphous nature of the as-quenched rods was confirmed by XRD. The rods were machined to specimens of 3mm in diameter and 10mm in length. The heating crystalline transition curves at a constant rate and the deformation under the isobaric pressure of 1N in the SLR at various heating rates for the alloy were measured with fully automatic instrument. The schematic diagram of the sample assembly is shown in Fig.1. The specimens were fixed in the quartz tube. The specimens change was fed to D.T core by quartz rod moving up or down along the differential transformer core(D.T core), which changes the output voltage of transformer, thus the dilation signal was transformed into electric signal. A differential transformer of very high sensitivity, stability, linearity and response, permits accurate measurement of even very small deformation.
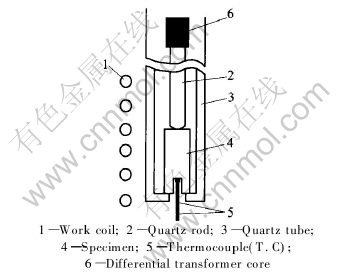
Fig.1 Schematic diagram of sample assembly
The deformation experiment was carried out in the vacuum of 10-4Pa and at different heating rates of 5, 10, 15, 20, 30, 60 and 100K/min respectively. The highest heating temperature was 650℃. The XRD patterns were obtained using D/MAX-γB diffractometer with graphite monochromater and CuKα radiation.
3 RESULTS AND DISCUSSION
Fig.2 shows the curves of the heating crystallization transition for the Zr41Cu12.5Ni10Be22.5 bulk amorphous alloy. It can be seen that glass transition temperature(Tg) increases slightly and the onset crystallization temperature(Tx) significantly increases with increasing heating rate, which causes the SLR(ΔT=Tg-Tx) larger. The crystallization temperature region(To-Tx) decreases with increasing heating rate. The super-cooled liquid region ΔT=Tg-Tx is a main factor when evaluating the stability of amorphous alloy, so increasing the SLR to some degree can increase the stability of amorphous alloy. And it provides conditions for studying the mechanical behavior and microstructure of bulk amorphous alloy in the SLR. As the heating rate increases from 5 to 60℃/min, the SLR(ΔT) increases from 35 to 105℃, and the crystallization temperature region decreases from 100 to 25℃. As the heating rate reaches 100℃/min, it is rather difficult to measure the crystallization temperature using dilatometry. The crystallization temperature of amorphous alloy approaches 500℃and ΔT reaches 115℃ by XRD.
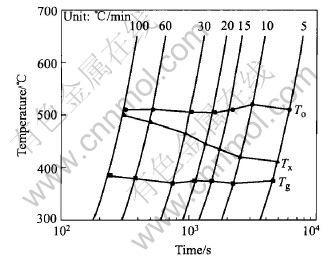
Fig.2 Curves of continuous heating crystallization transition curves of alloy
The deformation for the alloy under isobaric pressure at various heating rates in the SLR is shown in Fig.3. It is noted that the amount of deformation for the alloy in the SLR increases with increasing heating rate. As the heating rate increases from 5 to 100℃/min, the amount of deformation increases from 8.3% to 45%. Fig.4 shows XRD pattern for the alloy heated at 100℃/min to 500℃. The analysis shows that the crystallization phase in the alloy consists of a little Ti2Ni nanocrystal and amorphous phase.
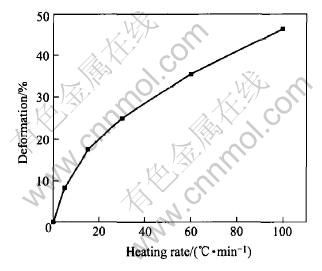
Fig.3 Deformation of alloy under same pressure at various heating rates in SLR
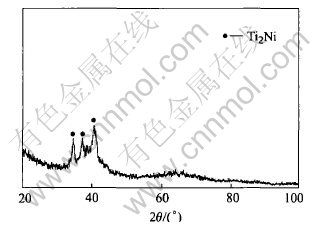
Fig.4 XRD pattern of alloy heated at 100℃/min to 500℃
The strength of the amorphous specimen is very high at the temperature below glass transition temperature, then the deformation under the pressure exerted by the quartz rod is neglected. As the temperature reaches the glass transition temperature, the specimens strength suddenly decreases owing to the effect of tension creep behavior of viscosity cropping up[20], so the deformation of specimens occurs. With increasing heating rate, owing to the asynchronism of the specimens surface and core temperature arriving at Tg and Tx, causing extra deformation of inside tension, the amount of deformation under the same pressure in the SLR becomes bigger. It is obvious that when outside force is a constant, the deformation ability of the amorphous alloy in the super-cooled liquid region increases with increasing heating rate. Using this characteristic, we can make the amorphous alloy become amorphous alloy ball and minisize gear wheel very smooth and light. The increase in the heating rate can increase not only the stability but also the amount of deformation, which improves the glass-processing behavior of the alloy.
REFERENCES
[1]Duwez P S, Lin C H. Amorphous ferromagnetic phases in iron-carbon phosphorus alloys [J]. App Phys, 1967, 38: 4096-4098.
[2]Zhang X Y, Guan Y, Yang L, et al. Crystallographic texture and magnetic anisotropy of α-Fe/Nd2Fe14B nanocomposites prepared by controlled melt spinning [J]. Appl Phys Lett, 2001, 79(15): 2426 - 2428.
[3]Zhang X Y, Zhang J W, Wang W K. A novel route for the preparation of nanocomposite magnets [J]. Advanced Materials, 2000, 12(19):1441-1444.
[4]Zhang X Y, Zhang F X, Zhang J W, et al. Influence of pressure on the crystallization process of an amorphous Fe73.5Cu1Nb3Si13.5B9 alloy [J]. J Appl Phys, 1998, 84: 1918-1923.
[5]Inoue A, Zhang T. Impact fracture energy of bulk amorphous Zr55Al10Cu30Ni5 alloy [J]. Materials Transactions JIM, 1996, 37(11):1726-1729.
[6]Nishiyama N, Inoue A. Glass-forming ability of bulk Pd40Ni10Cu30P20 alloy [J]. Materials Transactions JIM, 1996, 37(10):1531-1539.
[7]Inoue A. High strength bulk amorphous alloys with low critical cooling rates [J]. Mater Trans JIM, 1995, 36(5): 866-873.
[8]Johnson W L. Fundamental aspacts of bulk metallic glass formation in multicom ponents alloys [J]. Mater Sci Forum, 1996, 35(8): 225-227.
[9]Peker A, Johnson W L. A highly processable metallic glass: Zr41Ti14Cu12.5Ni10Be22.5 [J]. Appl Phys Lett,1993, 68(6): 2342-2344.
[10]Coehoorn R, de Mooij D B, Duchateau J P W B. Novel permanent magnetic material made by rapid quenching [J]. De Phys C 8 Supplemen, 1988, 49(2)E: 669-673.
[11]Kemeny T, Kaptas D, Bujdoso L, et al. Structure and magnetic properties of nanocrystalline(Fe1-xCox)90Zr7B2Cu1(0≤x≥0.6) [J]. Appl Phys Lett, 2000, 76(15)M: 2110-2112.
[12]Eckert J, Kubler A, Schultz L. Mechanically alloyed Zr55Al10Cu30Ni5 metallic glass composites containing nanocrystalline W particles [J]. Appl Phys, 1999, 85(10): 7112-7119.
[13]Nishiyana N, Inoue A. Glass transition behavior and viscous flow working of Pd40Cu30Ni10P20 amorphous alloy [J]. Materials Transactions JIM, 1999, 40(1): 64-71.
[14]Akatsuka R, Zhang T, Inoue A. Preparation of new Ni-based amorphous alloy with a large supercooled liquid region [J]. Materials Transactions JIM, 1999, 40(3): 258-261.
[15]Zhang T, Inoue A. Preparation of Ti-Cu-Ni-B amorphous alloy with a large supercooled liquid region [J]. Materials Transactions JIM, 1999, 40(4): 301-306.
[16]Frankwicj P S, Ram S, Fecht H J. Enhanced microhardness in Zr65.0Al7.5Ni10.0Cu17.5 amorphous rods on coprecipitation of nanocrystallites through supersaturated intermediate solid phase particles [J]. Appl Phys Lett, 1996, 68(20): 2825-2827.
[17]Peker A, Johnson W L. Bearing Amorphous Metallic Alloys Formed at Low Cooling Rates [P]. US 5288344, 1993.
[18]Wang W H. Microstructure, decomposition and crystallization in Zr41Ti14Cu12.5Ni10Be22.5 bulk metallic glass [J]. Phys Rev B, 1998, 57(5): 8211-8217.
[19]Zhang K Q, Chen Y, Zhang X Y. Crystallization kinetics of Zr41Ti14Cu12.5Ni10Be22.5 bulk metallic glass [J]. Trans Nonferrous Met Soc China, 2002, 12(2): 280-282.
[20]Guo Yi Ch, Wang Zh X. Amorphous Physics [M]. Beijing: Science Press, 1983. 315-326.
(Edited by YANG Bing)
Foundation item: Project(10174059) supported by the National Natural Science Foundation of China; Project(502171) supported by the Natural Science Foundation of Hebei Province, China
Received date: 2004-02-01; Accepted date: 2004-12-26


