
Performance of electrolyte with dimethyl methyl phosphonate as flame-retardant additive
ZHOU Dai-ying(周代营)1, LIU Jian-sheng(刘建生)2, 3, LI Wei-shan(李伟善)1,
TAN Chun-lin(谭春林)1, XU Meng-qing(许梦清)2, ZHAO Ling-zhi(赵灵智)1
1. Department of Chemistry, South China Normal University, Guangzhou 510006, China;
2. College of Materials Science and Engineering, South China University of Technology,Guangzhou 510641, China;
3. Guangzhou Tinci High New Material Technology Trade Co. Ltd., Guangzhou 510600, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
To improve the safety of lithium-ion batteries, dimethyl methyl phosphonate(DMMP) was used as a flame-retardant additive in a LiPF6 electrolyte solution. The flammability and thermal stability of DMMP-containing electrolyte was investigated by means of burning test and accelerating rate calorimeter. It was found that the flammability and self-heat rate of the electrolyte can be reduced by the addition of DMMP. On the other hand, the electrochemical performances of graphite/LiCoO2 cells become slightly worse after using DMMP additive in the electrolyte. This alleviated trade-off between electrolyte flammability, thermal stability, and cell performance provides a possibility to formulate a nonflammable electrolyte containing DMMP and improve the electrolyte thermal stability with a minimal sacrifice in performance.
Key words:
lithium ion batteries; dimethyl methyl phosphonate(DMMP); fame-retardant;
1 Introduction
Lithium ion batteries have been widely used, but their safety needs to be improved. The composition of electrolyte is essential for the performance improvement of lithium ion batteries[1-5]. However, the safety of the batteries is related to flammability of the electrolyte. Thus, flame retardancy is a major challenge for the safety improvement of lithium ion batteries[6]. Though much efforts have been devoted to formulating an electrolyte that is nonflammable and also works well in lithium ion batteries, the results have not been very satisfactory[7-9]. The attempt to improve the electrolyte flammability is always accompanied by the capacity loss of the batteries. The flame retardants that have been explored so far include the trimethyl phosphate(TMP) [6], hexamethoxycyclotriphosphazene(HMPN)[7], tri(β- chloromethyl)phosphate(TCEP)[8], tris(2,2,2-trifluotohyl) phosphate(TFP), bis (2,2,2-trifluotomethylphosphate (BMP) and (2,2,2-trifluotoethyl) diethyl phosphate (TDP)[9] and so on. Most of them are phosphate- containing compounds. With the application of these flame retardants, the electrolyte flammability is suppressed but the batteries suffer from severe capacity fading. This limitation is ascribed to the high viscosity of these retardants. Dimethyl methyl phosphonate(DMMP) is a phosphate containing compound but its viscosity is small compared with that of the phosphate-containing compounds mentioned above, which has good anti-flammability, thermal stability and compatibility with solvent. This compound was found to be an effective flame-retardant for lithium ion battery[10], but its effect on battery performance was not reported. The electrochemical and thermal stabilities and cyclic stability of the battery with DMMP as flame-retardant are considered in this work.
2 Experimental
To examine the effect of flammability of electrolyte, 5%, 10%, 15%, 20%, 30% and 40% of DMMP (mass fraction) were added directly to the 1 mol/L LiPF6/EC+ DMC+EMC electrolytes (where EC represents C3H4O3, DMC represents C3H6P3 and EMC is C4H8O3). The preparation of electrolytes was performed in an argon-filled glove box. Fiberglass wicks (4 cm in length, 8 mm in diameter) were first immersed in the electrolytes, absorbing about 1 g electrolyte and then set horizonically on the stand. A lighter was used to burn one end of the fiber, and a timer was used to record the burning time of the electrolytes. Each test was repeated seven times and the burning times recorded were averaged for the electrolyte samples containing different amount of these additives.
The conductivity measurements were taken at 25 ℃ using Model DDS-307A Conductometer (Digital) (Shanghai Precision Scientific Instrument Co. Ltd.). The constant was determined from measurement in a 0.01 mol/L KCl aqueous solution.
Voltammetry was carried out in a glass cell, which had separate compartments for the working electrode and the counter and reference electrodes. A glassy carbon disk (3.0 mm in diameter) electrode was used as working electrodes, respectively. Li foils were used as both reference and counter electrodes. The electrolyte was 1 mol/L LiPF6/EC+DMC+EMC.
The experimental prismatic lithium ion battery of 980 mA?h, which is nominally 6 mm thick, 34 mm wide and 48 mm long (Model 063448), was assembled using LiCoO2 cathode, graphite anode and polyethylene separator. Both cathodic and anodic electrodes were prepared through slurry-pasting and roll pressing. 5% of DMMP was added directly to the 1 mol/L LiPF6/EC+ DMC+EMC electrolyte. The preparation and injection of the electrolyte were conducted in argon filled globe box.
BK-7128L/2 lithium ion battery testing device (Guangzhou Blue-Key Electronic Industry Co. Ltd.) was used to test the cycle stability of the batteries. The batteries were charged and discharged with 1C constant current between 3.0 and 4.2 V.
The oven tests were conducted in the way that the battery was fully charged to 4.2 V after three circles and the oven temperature was elevated from environment temperature to 150 ℃, and then the temperature was held on. A thermal couple of model K was attached to the center of the largest face of the battery to record the temperature change of the battery through oven tests. By way of precaution, the oven tests on the prismatic batteries were conducted in a specially designed explosion resistant box. The thermocouple outputs from the test cells were connected to monitoring equipment outside the box.
The thermal stability of the electrolyte with and without the flame-retardant additive was investigated using an accelerating rate calorimeter (ARC), by ARC 2000, Arthur D Little Inc. For ARC studies, spherical bombs with Swagelok nuts and ferrules were used. Two samples were prepared: one contained 3 g of electrolyte and 10 mg of lithium metal, and the other contained 3 g of the electrolyte, 10 mg of lithium, and 150 mg of the flame-retardant additive. After preparing the samples in a dry glove box, the bombs were hermetically sealed and then transferred into the ARC. The ARC was programmed in heat wait search(HWS) mode to give a step increase of 10 ℃, and the starting and ending temperature were 30 ℃ and 270 ℃, respectively. The wait time was set at 40 min.
3 Results and discussion3.1 Physical properties
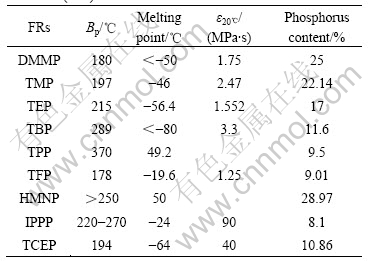
Table 1 summarizes the general physical properties of DMMP and the reported flame retardants. It can be seen from this Table that DMMP has lower boiling point, Bp (180 ℃) but higher phosphorus content (25%). It can be expected that the DMMP behaves better than others as flame-retardants.
Table 1 Physical properties of DMMP and reported flame retardants(FRs)
3.2 Electrochemical stability
The voltammograms of electrolytes with the flame- retardant additive in 1 mol/L LiPF6/EC+DMC+ EMC electrolyte are shown in Fig.1. It can be seen from Fig.1 that the DMMP does not decompose till 4.97 V. Thus the electrolytes containing this flame-retardant additive is safe for lithium ion batteries which operate between 2.5-4.3 V.
3.3 Flammability and ionic conductivity
The results of the electrolyte flammability test and the ionic conductivity(25 ℃) are shown in Fig.2. It can be seen that the base electrolyte, i.e. 1 mol/L LiPF6/EC+
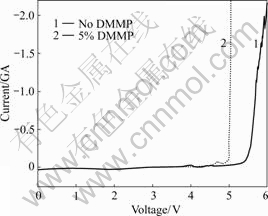
Fig.1 Voltammograms of glassy carbon electrode in 1 mol/L LiPF6/EC +DMC+ EMC (Scan rate: 1 mV/s)
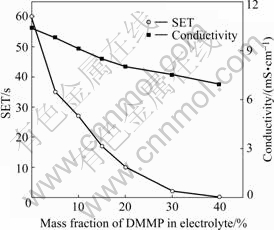
Fig.2 Effect of DMMP on flammability and ionic conductivity (25 ℃)of 1 mol/L LiPF6/EC+DMC+EMC electrolyte
DMC+EMC (1?1?1), is very flammable with self- extinguishing time(SET) of 60 s. As DMMP is added in the electrolyte, the SET greatly decreases to 35 s even with only 5% DMMP (in mass) and the electrolytes cannot be ignited (i.e. SET=0 s) when the content of DMMP exceeds 40% (in mass). Therefore, DMMP can efficiently suppress the flammability of the nonaqueous electrolyte. In general, organophosphorus compounds are known as flame retardants that can function in the vapor phase by a radical mechanism[11]. Usually the phosphorus content of this kind of flame-retardants can reflect the efficiency of flame retarding. DMMP has higher phosphorus content than the reported flame retardants such as trimethyl phosphate(TMP)[12], tributyl phosphate(TBP) and triphenyl phosphate(TPP) [13] so that it is more feasible to make the electrolyte become flame retardant or even totally nonflammable.
The possible reactions involved in the flame retarding process of DMMP in a fire situation can be expressed as Eqns.(1)-(3)[14-15]. DMMP gas is a good radical inhibitor by capturing the radicals H? and HO? in the flame zone so that it can weaken or terminate combustion chain branching reactions. H3PO4 can be decomposed further to produce the radicals PO? and PO2?, which can also capture H? and HO?. Furthermore, phosphorus acid can also function in the condensed phase. It can promote the char formation on the surface to insulate the substrate from heat and air, which can prevent the loss of decomposition products from the flame zone. The possible char formation mechanism can be expressed as Eqn.(4)[16]. As an adverse effect, it can be noticed from Fig.2 that the ionic conductivity of the electrolyte decreases with the increase of the DMMP content. This can be ascribed to the low dielectric constant of DMMP, which suppresses dissociation of electrolyte salt.
 (1)
(1)
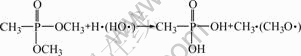 (2)
(2)
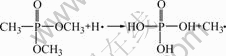 (3)
(3)
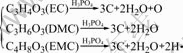 (4)
(4)
3.4 Cyclic stability
Fig.3 shows the cyclic stability of the cells with LiCoO2 and graphite as electrode materials. The DMMP-containing cell shows little decrease in capacity even after 150 cycles. This indicates that DMMP improve the safety of lithium-ion cells without significantly affecting its electrochemical performance.
3.5 Thermal performances
Fig.4 shows the self-heat rate profile of the electrolyte with and without the flame-retardant additive in the ARC. The maximum self-heat rate of the electrolyte without the flame-retardant additive is 0.70 ℃/min, which occurs at T=177.1 ℃. This can be attributed to the reaction of lithium metal with the electrolyte. As the reaction proceeds, the lithium metal is
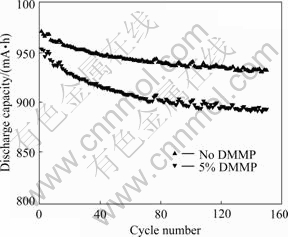
Fig.3 Cyclic stability of batteries with and without DMMP

Fig.4 Thermal behavior of electrolytes with and without DMMP
consumed, and thus the exothermic peaks decrease as the temperature increases beyond 177.6 ℃. On the other hand, the maximum self-heat rate of the electrolyte with the flame-retardant additive is only 0.232 ℃/min at T= 181 ℃. The peaks were suppressed in comparison with that for the electrolyte without the flame-retardant additive, which may be attributed to passivation layer that is formed on the surface of the lithium metal by the flame-retardant additive. Results of these thermal investigations strongly suggest that the addition of the flame-retardant additive in the electrolyte significantly reduces the self-heat rate. The reduced self-heat rate, in turn, helps in improving the nonflammability of the electrolyte.
Fig.5 shows the variation of the temperature of 063448 batteries with healing time. Without DMMP, when heated for 28 min, the surface temperature of the battery without DMMP started to rise dramatically at about 168 ℃. This happens for the battery containing DMMP when heated for 32 min. This verifies that the DMMP can improve the safety of the battery.
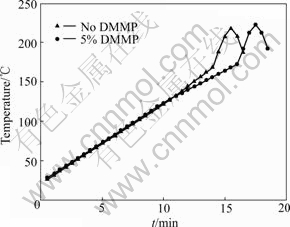
Fig.5 Variation of surface temperature of cell with time
4 Conclusions
1) The electrolyte containing the flame-retardant additive, dimethyl methyl phosphonate(DMMP), is electrochemically stable up to 4.97 V (vs Li/Li+) and hardly has negative effect on battery performances.
2) DMMP can reduce self-heat rate, and therefore improve the thermal stability and the nonflammability of the electrolyte.
References
[1] LU Lei, ZUO Xiao-xi, XU Meng-qing, LIU Jian-sheng, LI Wei-shan. A Study on the preparation and performances of PMMA-Vac electrolyte for lithium ion battery use [J]. Acta Chim Sinica, 2007, 65: 475-480. (in Chinese)
[2] ZHANG Zhong, XU Xuan, ZUO Xiao-Xi, LI Wei-Shan. Stabilizing effect of amine on small molecules in electrolyte of lithium batteries [J]. Acta Physico-Chimical Sinica, 2007, 23: 526-530. (in Chinese)
[3] ZUO Xiao-xi, XU Meng-qing, LI Wei-shan, SU Da-gen, LIU Jian-sheng. Electrochemical reduction of 1,3-propane sultone on graphite electrode and its application in Li-ion battery [J]. J Electrochem. Solid-State Lett, 2006, 9: 196-199.
[4] XU Meng-qing, ZUO Xiao-xi, LI Wei-shan, ZHOU Hao-jie, LIU Jian-sheng, YUAN Zhong-zhi. Effect of butyl sultone on the Li-ion battery performance and interface of graphite electrode [J]. Acta Physico-Chimical Sinica, 2006, 22(3): 335-340.
[5] LU Dong-sheng, LI Wei-shan, ZUO Xiao-xi, YUAN Zhong-zhi, HUANG Qi-ming. A study on kinetics of Li+ insertion/de-insertion in LixMn2O4 (0≤x≤1) by electrochemical impedance spectroscopy [J]. J Phy Chem C, 2007, 111: 12067-12074.
[6] XU Kang. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries [J]. Chem Rev, 2004, 104: 4400-4405.
[7] LEE C W, VENKATACHALAPATHY R, PRAKASH J. A novel flame-retardant additive for lithium batteries [J]. J Electrochem Solid-State Lett, 2000, 3(2): 63- 65.
[8] HE Y B, LIU Q, TANG Z Y, CHEN Y H, SONG Q S. The cooperative effect of tri(β-chloromethyl) phosphate and cyclohexyl benzene on lithium ion batteries [J]. Electrochimica Acta, 2007, 52(11): 3534-3540.
[9] XU K, ZHANG S S, ALLEN J L, JOW T R. Evalution of flurinated alkyl phosphates as flame retardants in electrolytes for Li-ion batteries (Ⅰ): Physical and electrochemical properties) [J]. J Electrochem Soc, 2003, 150(2): 161-169.
[10] XIANG H F, XU H Y, WANG Z Z, CHEN C H. Dimethyl methylphosphonate(DMMP) as an efficient flame retardant additive for the lithium-ion battery electrolytes [J]. J Power Sources, 10.1016/j.jpowsour.2007.05.001.
[11] OU Y X, Flame retardant—manufacture, performance and application [M]. Beijing: Weapon Industry Press, 1997: 87-88. (in Chinese)
[12] WANG X M, YASUKAWA E, KASUYA S. Nonflammable trimethyl phosphate solvent-containing electrolytes for lithium-ion batteries [J]. J Electrochem Soc, 2001, 148: 1058-1065.
[13] HYUNG Y E, VISSERS D R, AMINE K. Flame-retardant additives for lithium-ion batteries [J]. J Power Sources, 2003, 119: 383-387.
[14] KOROBEINICHEV O P, ILYIN S B, SHVARTSBERG V M, CHERNOV A A. The chemistry of the destruction of organophosphorus compounds in flames (III): The destruction of DMMP and TMP in a flame of hydrogen and oxygen [J]. Combustion and Flame, 2000, 121: 593-609.
[15] WERNER J H, COOL T A. Kinetic model for the decomposition of DMMP in a hydrogen/oxygen flame [J]. Combustion and Flame, 1999, 117: 78-98.
[16] WANG Q S, SUN J H, YAO X L, CHEN C H. 4-Isopropyl phenyl diphenyl phosphate as flame-retardant additive for lithium-ion battery electrolyte [J]. J Electrochemical and Solid-State Lett, 2005, 8: 467-470.
(Edited by LAI Hai-hui)
Foundation item: Projects(2006A10704003; 2006Z3-D2031) supported by the Key Project of Guangdong Province and Guangzhou City, China
Corresponding author: LI Wei-shan; Tel/Fax: +86-20-39310256; E-mail: liwsh@scnu.edu.cn


