
Deposition of photocatalytic TiO2 and N-doped TiO2 films by arc ion plating
LI Ming-sheng(李明升), ZHANG Shu-juan(张淑娟), LOU Jin(娄 瑾), LIU Ting-zhi(刘庭芝),
ZHOU Ze-hua(周泽华), YANG Gan-lan(杨干兰), HU Chang-yuan(胡长员), LI Wen-kui(李文魁)
Jiangxi Key Laboratory of Surface Engineering, Jiangxi Science and Technology Normal University, Nanchang 330013,China
Received 15 July 2007; accepted 10 September 2007
Abstract:
TiO2 and N-doped TiO2 films were deposited on the glass substrates by arc ion plating method. The results show that the deposition rate does not change with the increasing deposition time. The increase of mass flow rate of N2 gives rise to the increase of deposition rate. All as-deposited TiO2 and N-doped TiO2 films are amorphous. The anatase TiO2 phase with preferred orientation (101) is acquired by post-annealing at 400 ℃ for 2 h. The incorporation of N into the TiO2 films and the heat treatment extensively shift the band edge to the visible light region.
Key words:
arc ion plating; photocatalytic TiO2; N-doped TiO2; absorption edge;
1 Introduction
Photocatalytic TiO2 has been attracting more and more attention due to its high stability and activity, and can be used in many fields such as the decomposition of organic pollutant, splitting water and development of self-cleaning glass[1-3]. Since the band gap of TiO2 is approximately 3.2 eV, only the UV light of wave length less than 380nm can be absorbed[4]. Recently, many studies have been performed to extend the available spectrum for TiO2 from UV region to visible region. One approach in the development of such visible light-responsive catalysts was to develop various photosensitizing dyes adsorbed on TiO2 films[4-5] and another approach was to dope transition metal such as V[6], Cr[7], Fe[8], Co[9] and Ni[10] or anion such as carbon[11], nitrogen[12-13] and sulfur[14] into TiO2 films or particles. Photocatalytic TiO2 films can be prepared by several methods such as sol-gel[15], CVD[16], magnetron sputtering[17] and arc ion plating[18]. Owing to the high deposition rate, convenient parameter control and excellent film quality, arc ion plating is one of the most prospective methods to deposit TiO2 and anion-doped TiO2[19].
2 ExperimentalThe TiO2 and N-doped TiO2 films were deposited on microscope glass slides (with dimensions of 75.5 mm×25.5 mm×1.2 mm) by arc ion plating (AIP-1000- 10 Coating System). The glass slides were prepared by ultrasonic cleaning in acetone and then being blown to dry. The disc target was titanium with a purity of 99.95% and the target dimensions were 100 mm in diameter and 45 mm in thickness. The base pressure in the chamber was 2×10-3 Pa and the substrate temperature was room temperature at the beginning of the deposition. The reactive gases O2 (99.99%) and N2 (99.999%) were controlled by mass flowmeter. The total pressure of O2 and N2 was kept at 1.2 Pa. When TiO2 films were deposited, no N2 was introduced and the mass flow rate of O2 was 340 cm3/min. In order to get N-doped TiO2 films with different N contents, the mass flow rate of N2 was respectively set at 40, 80, 120, 160, 200, 240, 260 and 280 cm3/min. No bias voltage was applied to the substrates during the deposition of the films. The distance between target and substrate was 200 mm. The arc current was 40 A. Three kinds of TiO2 films with different thickness were prepared by controllingdeposition time at 15, 30 and 45 min. The deposition time of all N-doped TiO2 films was kept at 30 min. The film specimens were annealed at 400 ℃ for 2 h in a chamber-type electric furnace under atmospheric ambience.
The crystalline structure and crystallographic orientation of the films were measured by Bruker D8 ADVANCE X-ray diffractometer (XRD) using Cu Kα radiation. The surface morphology of the films was examined using a field emission SEM. UV-Vis spectra of the films on glass substrate were measured in air at room temperature using UNICO UV/VIS 2802PCS spectrophotometer in the range from 190 nm to 1 100 nm. The film thickness was determined by fitting transmittance optical spectra using Scout software.
3 Results and discussion3.1 Deposition rate
The thickness of the as-deposited TiO2 with deposition time of 15, 30 and 45 min is respectively 0.53, 1.05 and 1.57 μm. The deposition rate is about 35 nm/min for all these TiO2 films and keeps constant with the increasing deposition time. Fig.1 shows the curves of deposition rate versus the mass flow rate of N2. The deposition rate increases with the increase of mass flow rate of N2. This change of the deposition rate is ascribed to the change of evaporation rate of the Ti target in different reactive atmosphere.
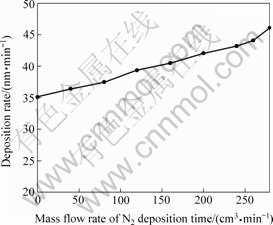
Fig.1 Curves of deposition rate of TiO2 films versus mass flow rate of N2
3.2 Phase structure
The XRD patterns of the as-deposited TiO2 films and the post-annealed specimens after different deposition time are shown in Fig.2. No distinct diffraction peak is detected in the results of all the three kinds of as-deposited films, thus indicating that all the TiO2 films are amorphous. Due to the high ionized rate and heating effect of plasma on the substrates, rutile phase is apt to form during the deposition was process. In order to prevent the formation of rutile phase structure, in this experiment, the deposition was processed under room temperature, the arc current was controlled at 40 A, and no bias voltage was applied to the substrates. Under this condition of lower energy, neither rutile phase nor anatase one can be formed. Heat treatment at 400 ℃ in air for 2 h was applied to the as-deposited TiO2 in order to get the complete anatase TiO2 phase. For the post-annealed films, as our anticipation, only the diffraction peaks of anatase TiO2 is found. (101) is the main preferential orientation. With the increasing thickness of the films, there exists an increasing intensity of the diffraction peaks for the post-annealed films.
Fig.3 shows the XRD patterns of the as-deposited N-doped TiO2 films deposited under different mass flow rate of N2. All the as-deposited films were amorphous.

Fig.2 XRD patterns of TiO2 films: (a) Post-annealed film, 45 min deposition time; (b) Post-annealed film, 30 min deposition time; (c) Post-annealed film, 15 min deposition time; (d) As-deposited film, 45 min deposition time; (e) As-deposited film, 30 min deposition time; (f) As-deposited film, 15 min deposition time

Fig.3 XRD patterns of as-deposited N-doped TiO2 films under different mass flow rates of N2
The N-doped TiO2 films were annealed at 400 ℃ in atmospheric ambience to acquire crystalline films. Fig.4 shows the XRD patterns of the post-annealed N-doped TiO2 films. Unexceptionally, all the post-annealed films are anatase with a (101) preferential orientation.

Fig.4 XRD patterns of post-annealed N-doped TiO2 films different flow rates of N2
3.3 Optical properties
TiO2 film deposited for 15 min or 30 min was colorless and transparent to visible light. While the film deposited for 45 min is not complete transparent to visible light. Fig.5 shows the UV-Vis spectra of the as-deposited and the post-annealed TiO2 after different deposition time. The band edges of the deposited films shift towards the visible region obviously with the increasing deposition time. This shift of band edges is ascribed to the quantum size effect[20]. The transmittance of the as-deposited TiO2 films decreases with increasing deposition time and this is due to the increase of the film thickness. Annealing treatment hardly changes the band edges of the film deposited for 15 min or 30 min, but the post-annealed film deposited for 45 min exhibits considerable absorption in wavelength regions longer than 400 nm. The transmittance of the films decreases to some extent owing to the post-annealing. With increasing thickness of the films, the transmittance decreases more significantly.
Fig.6 shows the UV-VIS spectra of the as-deposited and post-annealed N-doped TiO2 films deposited under different mass flow rates of N2. For the as-deposited films, the introduction of N into the films gives rise to a distinct shift of band edges to visible region and a constant decrease of the transmittance. The post-annealing further makes the band edges shift to longer wavelength. It is reported that most of N-doped TiO2 exhibits visible light absorption as a shoulder in the wavelength range of 400–600 nm, which indicates that the isolated N 2p orbital are formed above the O 2p orbital due to the limited concentration of N that the substitute for O in TiO2 lattice is lower than 2%[21]. But in this work, even the N-doped TiO2, whose band edge is close to 500 nm, does not show the visible light absorption as a shoulder. Compared with other deposition technique such as sol-gel and magnetron sputtering, arc ion plating method possesses high ionization rate, N can be easily introduced to the lattice of TiO2. The mixing of N 2p states with the O 2p states shifts the valence band edge upwards to narrow the band gap energy of TiO2.

Fig.5 UV-vis transmission spectra of as-deposited and post-annealed TiO2 films after different deposition time: 1—15 min, as-deposited; 2—30 min, as-deposited; 3—45 min, as-deposited; 4—15 min, post-annealed; 5—30 min, post-annealed; 6—45 min, post-annealed

Fig.6 UV-vis transmission spectra of as-deposited and post- annealed N-doped TiO2 films under different flow rates of N2: 1—0 cm3/min; 2—120 cm3/min; 3—200 cm3/min; 4—240 cm3/ min; 5—260 cm3/min; 6—0 cm3/min, post-annealed; 7—120 cm3/min, post-annealed; 8—200 cm3/min, post-annealed; 9—240 cm3/min, post-annealed; 10—260 cm3/min, post-annealed
4 Conclusions1) TiO2 and N-doped TiO2 films were deposited on glass substrates by arc ion plating technique. The thickness of the TiO2 films is linear with the deposition time. The increase of mass flow rate of N2 improves the deposition rate of the films. All as-deposited TiO2 and N-doped TiO2 films are amorphous.
2) After annealed at 400 ℃ for 2 h, the anatase TiO2 phase with (101) preferred orientation is acquired. The introduction of N into the TiO2 lattice narrows the band gap energy of TiO2 and gives rise to a shift of the band edge to the visible light region. The post-annealing further makes the band edges shift to longer wavelength.
References
[1] WATANABE T, NAKAJIMA A, WANG R, MINABE M, KOIZUMI S, FUJISHIMA A AND HASHIMOTO K. Photocatalytic activity and photoinduced hydrophilicity of titanium dioxide coated glass[J]. Thin Solid Films, 1999, 351(1/2): 260-263.
[2] HARADA H. Sonophotocatalytic decomposition of water using TiO2 photocatalyst[J]. Ultrasonics Sonochemistry, 2001, 8: 55-58
[3] HATA S, KAI Y, YAMANAKA I,OOSAKI H,KAZUO HIROTA,YAMAZAKI S. Development of hydrophilic outside mirror coated with titania photocatalyst[J]. JSAE Review, 2000, 21(1): 97-102.
[4] KITANO M, MATSUOKA M, UESHIMA M, ANPO M. Recent developments in titanium oxide-based photocatalysts[J]. Applied Catalysis A: General, 2007, 325: 1-14.
[5] MURAKOSHI K, KANO G, WADA Y, YANAGIDA S, MIYAZAKI H, MATSUMOTO M, MURASAWA S. Importance of binding states between photosensitizing molecules and the TiO2 surface for efficiency in a dye-sensitized solar cell[J]. Journal of Electroanalytical Chemistry, 1995, 396(1): 27-34.
[6] GAO Y, THEVUTHASAN S, MCCREADY D E, ENGELHARD M. MOCVD growth and structure of Nb- and V-doped TiO2 films on sapphire[J]. Journal of Crystal Growth, 2000, 212(1): 178-190.
[7] SHARMA R K, BHATNAGAR M C, SHARMA G L. Mechanism of highly sensitive and fast response Cr doped TiO2 oxygen gas sensor[J]. Sensors and Actuators B: Chemical, 1997, 45(3): 209-215.
[8] PAN Xiao-yan, JIANG Dong-mei, LIN Yan, MA Xue-ming. Structural characterization and ferromagnetic behavior of Fe-doped TiO2 powder by high-energy ball milling[J]. Journal of Magnetism and Magnetic Materials, 2006, 305(2): 388-391.
[9] IWASAKI M, HARA M, KAWADA H, TADA H, ITO S. Cobalt ion-doped TiO2 photocatalyst response to visible light[J]. Journal of Colloid and Interface Science, 2000, 224(1): 202-204.
[10] GU X, WANG C. Effect of nickel ion doping on photocatalytic activity of TiO2 films[J]. Journal of the Chinese Ceramic Society 2004, 32(5): 558-563. (in Chinese)
[11] IRIE H, WASHIZUKA S, HASHIMOTO K. Hydrophilicity on carbon-doped TiO2 thin films under visible light[J]. Thin Solid Films, 2006, 510(1-2): 21-25.
[12] SUDA Y, KAWASAKI H, UEDA T, OHSHIMA T. Preparation of high quality nitrogen doped TiO2 thin film as a photocatalyst using a pulsed laser deposition method[J]. Thin Solid Films, 2004, 453/454: 162-166.
[13] IRIE H, WATANABE Y, HASHIMOTO K. Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2-xNx Powders[J]. J Phys Chem B, 2003, 107(23): 5483-5486.
[14] NISHIJIMA K, NAITOH H, TSUBOTA T, OHNO T. Visible-light-induced hydrophilic conversion of an S-doped TiO2 thin film and its photocatalytic activity for decomposition of acetaldehyde in gas phase[J]. Journal of the Ceramic Society of Japan, 2007, 115(1341): 310-314.
[15] PECCHI G, REYES P, SANHUEZA P, VILLASE?OR J. Photocatalytic degradation of pentachlorophenol on TiO2 sol-gel catalysts[J]. Chemosphere, 2001, 43(2): 141-146.
[16] MATHUR S, KUHN P. CVD of titanium oxide coatings: Comparative evaluation of thermal and plasma assisted processes[J]. Surface and Coatings Technology,2006, 201(3/4): 807-814.
[17] TAKEDA Satoshi, SUZUKI Susumu, ODAKA Hidefumi, HOSONO Hideo. Photocatalytic TiO2 thin film deposited onto glass by DC magnetron sputtering[J]. Thin Solid Films, 2001, 392(2):338-344.
[18] YUMOTOA H, MATSUDOA S, AKASHIB K. Photocatalytic decomposition of NO2 on TiO2 films prepared by arc ion plating[J]. Vacuum, 2002, 65: 509-514.
[19] LI Ming-sheng, WANG Fu-hui. Effects of nitrogen partial pressure and pulse bias voltage on (Ti,Al)N coatings by arc ion plating[J]. Surface and Coatings Technology,2003, 167(2/3): 197-202.
[20] ZHANG Wen-jie, ZHU Sheng-long, LI Ying, WANG Fu-hui. Photocatalytic property of TiO2 films deposited by pulsed DC magnetron sputtering[J]. J Mater Sci Technol, 2004, 20(1): 31-34.
[21] KITANO M, MATSUOKA M, UESHIMA M, ANPO M. Recent developments in titanium oxide-based photocatalysts[J]. Applied Catalysis A, 2007, 325: 1-14.
Foundation item: Project (50401022) supported by the National Natural Science Foundation of China; Project (0650034) supported by the Natural Science Foundation of Jiangxi Province, China
Corresponding author: LI Ming-sheng; Tel: +86-791-3831266; E-mail:mshli@163.com


