
Synthesis of LiNi0.8Co0.1Mn0.1O2 cathode material by chloride co-precipitation method
LI Ling-jun(李灵均), LI Xin-hai(李新海), WANG Zhi-xing(王志兴),
WU Ling(伍 凌), ZHENG Jun-chao(郑俊超), LI Jin-hui(李金辉)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 6 July 2009; accepted 30 December 2009
_____________________________________________________________________________________________________
Abstract:
LiNi0.8Co0.1Mn0.1O2 was prepared by a chloride co-precipitation method and characterized by thermogravimetric analysis, X-ray diffractometry with Rietveld refinement, electron scanning microscopy and electrochemical measurements. Effects of lithium ion content and sintering temperature on physical and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 were also investigated. The results show that the sample synthesized at 750 ℃ with 105% lithium content has fine particle sizes around 200 nm and homogenous sizes distribution. The initial discharge capacity for the powder is 184 mA?h/g between 2.7 and 4.3 V at 0.1C and room temperature.
Key words:
lithium ion battery; LiNi0.8Co0.1Mn0.1O2; chloride co-precipitation; Rietveld refinement;
_____________________________________________________________________________________________________
1 Introduction
Recently, layered LiNi0.8Co0.1Mn0.1O2 has been intensively studied as a potential positive active electrode for application in batteries for hybrid electric vehicles (HEVs)[1-2]. It is reported that the mixed oxide inherits the merits of mono metal oxide such as LiCoO2, LiNiO2 and LiMnO2, and exhibits high capacity, good cycling stability and excellent safety performance[3-6]. Nevertheless, compared with other lithium ion cathode materials, such as LiFePO4, the cycling ability of LiNi0.8Co0.1Mn0.1O2 still needs to be improved, due to irreversible capacity loss caused by cation mixing[2, 7-9]. Several effective ways have been proposed to lower the mixing degrees, including adding excess Li ion to restrain Ni2+ moved to Li layer, and substitution of a small quantity of layered metal by Al and Mg ions[2, 10-11].
The tradition way to synthesize LiNi0.8Co0.1Mn0.1O2 is co-precipitation method with sulphate and nitrate as raw materials, and their particle sizes are 10-40 μm[9, 12]. As pointed out before, the electrochemical behavior of lithium ion cathode material is strongly influenced by preparation method, which includes different raw materials and particle size of final product[13-14]. It is also reported that submicron and nanometer particles are conducive to shorten the Li ion diffusion route, and finally improve the electrochemical performance of material[15]. Therefore, we assumed that adding excessive Li and decreasing particle size maybe benefit the capacity and cycling-life of LiNi0.8Co0.1Mn0.1O2. In this work, through a chloride co-precipitation method, submicron LiNi0.8Co0.1Mn0.1O2 was attained, and effects of lithium ion content and sintering temperature on physical and electrochemical performance of final products were also investigated.
2 ExperimentalLiNi0.8Co0.1Mn0.1O2 was synthesized with the starting materials of LiOH·H2O, NiCl2·6H2O, CoCl2·6H2O, MnCl2·4H2O, NaOH and NH3·H2O. NiCl2·6H2O, CoCl2·6H2O and MnCl2·4H2O powders were dissolved in distilled water to obtain 2 mol/L solution (n(Ni):n(Co):n(Mn)=8:1:1). The mixtures were filled in beaker and heated at 50 ℃ in water bath kettle, then NH3·H2O and 2 mol/L NaOH were added to the solution to control the pH value between 11 and 12. After being stirred for 5 min, the solution was filtered to separate the precipitate powders, and the powder was washed with distilled water and dried at 100 ℃ for 8 h. Later, LiOH was milled with the powder for 0.5 h in stoichiometric ratios of Li to (Ni+Co+Mn) is 1.03, 1.05 and 1.07, respectively, and the obtained mixture was sintered at 480 ℃ for 5 h, and then sintered at 750 ℃ for 12 h in O2 atmosphere, respectively. After being cooled to room temperature, the finale samples were obtained.
The TG—DTG analysis was tested by SDTQ600 with a step time of 10 (?)/min in O2 atmosphere. The crystalline nature of the samples was identified with X-ray diffractometry (XRD, Dmax/2550VB+18 kW, Rigaku using monochromatic Cu Kα radiation with a step time of 2(?)/min), and X-ray Rietveld re?nement was performed by FULLPROF. Powder morphologies were observed by scanning electron microscope (SEM, JEOL JSM- 5600LV).
The electrochemical properties of LiNi0.8Co0.1Mn0.1O2 were measured by using 2025 button cell. The cathode consisted of 80% (mass fraction) active material, 10% acetylene black and 10% PVDF binder. A lithium metal foil was used as anode. LiPF6 (1 mol/L) in a 1:1:1(volume ratio) mixture of dimethyl carbonate (DMC), ethyl methyl carbonate (EMC) and ethylene carbonate (EC) was used as electrolyte. Charge– discharge performances were evaluated using a battery test system (BTS-5V/1mA, Xinwei 2.7-4.3 V).
3 Results and discussionThe TG—DTG curves of the mixture of LiOH·H2O and Ni0.8Co0.1Mn0.1(OH)2 powders are shown in Fig.1. It is clear that three endothermic peaks and mass loss sections appear at 80-96℃, 226-273℃, 430℃, respectively, which represent the loss of absorbed water, dehydration reactions of LiOH·H2O and Ni0.8Co0.1- Mn0.1(OH)2, respectively. With the increase of temperature, the dehydration products, Ni0.8Co0.1Mn0.1O2 and Li2O, react slowly to LiNi0.8Co0.1Mn0.1O2. Owing to the fact that the reaction is composed by several sections including dehydration process as shown above, two- section sinter should be introduced to ensure sufficient decomposition and contact between LiOH·H2O and precursor, and finally attain well electrochemical performance.
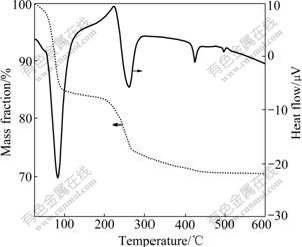
Fig.1 TG—DTG curves of mixture of LiOH·H2O and Ni0.8Co0.1Mn0.1(OH)2 powders
Fig.2 shows the XRD patterns of LiNi0.8Co0.1- Mn0.1O2, synthesized at different temperatures or Li ion content. It is noted that all diffraction lines are indexed on the basis of the rhombohedral α-NaFeO2 structure with a space group of R-3m. The samples with 105% Li ion content in Fig.2(a) were prepared at 700, 750 and 800 ℃, respectively. It is obvious that the ratio of I003 to I104 for LiNi0.8Co0.1Mn0.1O2 powder obtained at 750℃ is 1.51, larger than 1.01 and 1.20 obtained at 700 ℃ and 800℃, respectively, which scales lower mixing degree of Ni+ in Li layer than others. As we know, the excess of Li+, whether in Li layer or in transition metal layer, will suppress the migration of Ni+ into Li layer. Fig.2(b) shows the effect of various Li+ contents on the XRD pattern of LiNi0.8Co0.1Mn0.1O2 powders prepared at 750 ℃. It is found that the ratio of I003 to I104 for LiNi0.8Co0.1Mn0.1O2 powders is gradually enhanced from 1.29, 1.51 , to 1.52 with the increase of Li ion content.
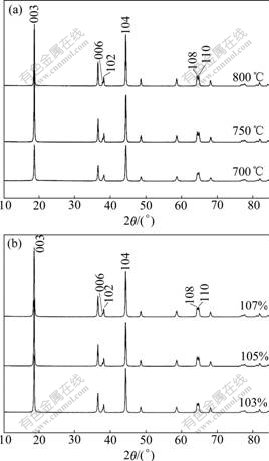
Fig.2 XRD patterns of LiNi0.8Co0.1Mn0.1O2, synthesized at different temperatures (a) and Li ion contents (b)
The corresponding Rietveld refinement plot and parameters of LiNi0.8Co0.1Mn0.1O2 powder synthesized at 750 ℃ are shown in Fig.3 and Table 1, respectively.The refinements were performed by assuming a partial occupation of Ni in the Li layers. The refinement results are in good agreement between the observed and calculated patterns in Fig.3 with high reliability factor. As seen in Table 1, only 5.5% Ni moves to the 3b-site (lithium site), and the lattice parameters of LiNi0.8Co0.1Mn0.1O2 are smaller compared with the results from other research groups. WOO et al[11] reported that a=0.286 87 nm, c=1.425 31 nm, which demonstrated that well-ordering layered and smaller LiNi0.8Co0.1Mn0.1O2 powder can be prepared by chloride co-precipitation method.
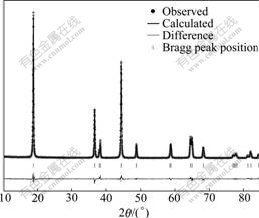
Fig.3 Rietveld refinement patterns of XRD data for LiNi0.8Co0.1Mn0.1O2 (synthesized with 105% Li ion content at 750 ℃)
Table 1 Structural parameters obtained from Rietveld Refinement of XRD data of LiNi0.8Co0.1Mn0.1O2

Fig.4 presents the SEM images of the precursor and LiNi0.8Co0.1Mn0.1O2 powder synthesized with 105% of Li content at 750 ℃. As it can be seen that the particle morphologies of images are near-spherical and well distributed. And the particle sizes of precursor and LiNi0.8Co0.1Mn0.1O2 powders are fine, about 200 nm, which is conducive to shorten the lithium diffusion distance.
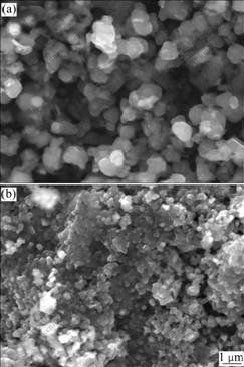
Fig.4 SEM images of Ni0.8Co0.1Mn0.1(OH)2 and LiNi0.8Co0.1- Mn0.1O2
The initial discharge curves of LiNi0.8Co0.1Mn0.1O2 prepared at different temperatures and Li ion contents are shown in Fig.5. The batteries were measured at about 10 ℃, 2.7-4.3 V and 0.1C (18 mA?h/g), respectively. All the samples exhibit a diagonal voltage plateau from 4.3 V to 3.58 V. In Fig.5, the sample obtained at 750 ℃ with 105% of Li ion content deliveries the best discharge capacity, 184 mA?h/g, which is consistent with that shown in Fig.2 or Fig.3, that lower mixing degree of Ni+ in Li layer results in better electrochemical performance. It is also noted that the capacities of sample synthesized with 107% and 103% are almost equal, which can be ascribed that too much Li ion might occupy the site of transition metals, while, the lack of Li ion might induce to cation mixing, both of which should take responsibility to the capacity irreversible loss of LiNi0.8Co0.1Mn0.1O2.
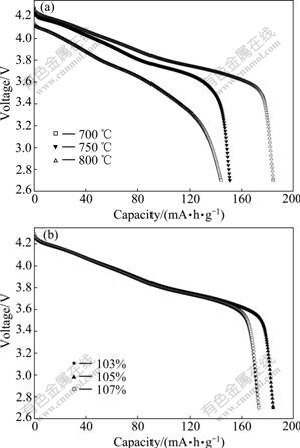
Fig.5 First discharge curves of LiNi0.8Co0.1Mn0.1O2 synthesized at different temperatures (a) and Li ion contents (b) at 18 mA?h/g (0.1C) and 2.7-4.3 V
Fig.6 shows the cycling abilities of LiNi0.8Co0.1Mn0.1O2 synthesized with different Li ion contents. After 30 cycles, all samples experience various capacity loss degrees. The sample prepared with 103% of Li ion content exhibits the worst cycle ability, and its discharge capacity was only 140 mA?h/g after 30 cycles. The capacity of sample prepared with 107% of Li ion content, on the other hand, exhibits 149 mA?h/g after 30 cycles. This demonstrated that excess Li ion can avoid cation mixing and improve cycle ability effectively. The sample prepared with 105% of Li ion content is found to be the optimum, which exhibited the highest discharge capacity and well cycle-life, with 169 mA?h/g after 30 cycles.
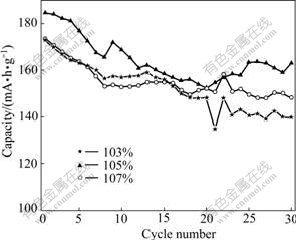
Fig.6 Cycling performance of LiNi0.8Co0.1Mn0.1O2 synthesized with various Li ion content tested at 0.1C and 2.7- 4.3 V
4 Conclusions
1) Layered LiNi0.8Co0.1Mn0.1O2 compounds are synthesized with various Li ion contents at different temperatures by a chloride co-precipitation method.
2) XRD, Rietveld refinement and SEM analyses indicate that well-ordering, near-spherical and well distributed precursor has attained, and the particle size of LiNi0.8Co0.1Mn0.1O2 compound is nearly 200 nm.
3) It is noted that the initial discharge capacity of the LiNi0.8Co0.1Mn0.1O2 synthesized with 105% of Li ion content at 750 ℃ is 184 mA?h/g, showing 169 mA?h/g after 30 cycles.
References[1] KIM M H, SHIN H S, SHIN D, SUN Y K. Synthesis and electrochemical properties of LiNi0.8Co0.1Mn0.1O2 and LiNi0.8Co0.2O2 via co-precipitation [J]. Power Sources, 2006, 159(2): 1328-1333.
[2] EOM J H, KIM M G, CHO J. Storage characteristics of LiNi0.8Co0.1+xMn0.1-xO2 (x=0, 0.03, and 0.06) cathode materials for lithium batteries [J]. Electrochem Soc, 2008, 155(3): 239-245.
[3] OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries [J]. Chem Lett, 2001, 30(7): 642-643.
[4] SUN Y K, MYUNG S T, PARK B C. Synthesis of spherical nano- to microscale core shell particles Li[(Ni0.8Co0.1Mn0.1)1-x(Ni0.5Mn0.5)x]O2 and their applications to lithium batteries [J]. Chem Mater, 2006, 18(22): 5159-5163.
[5] HIRANO A, KANNO R, KAWAMOTO Y. Neutron diffraction study of LiNi0.8Mn0.2O2 [J]. Solid State Chem, 1997, 134(1): 1-4.
[6] KOSOVA N V, DEVYATKINA E T, KAICHEV V V. Optimization of Ni2+/Ni3+ ratio in layered Li(Ni, Mn, Co)O2 cathodes for better electrochemistry [J]. Power Sources, 2007, 174(2): 965-969.
[7] PADHI A K, NAJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. Electrochem Soc, 1997, 144(4): 1188-1194.
[8] LI L J, LI X H, WANG Z X, WU L, ZHENG J C, GUO H J. Stable cycle-life properties of Ti-doped LiFePO4 compounds synthesized by co-precipitation and normal temperature reduction method [J]. Phys Chem Solids, 2009, 70(1): 238-242.
[9] KIM H B, PARK B C, MYUNG S T, AMINE K, PRAKASH J, SUN Y K. Electrochemical and thermal characterization of AlF3-coated Li[Ni0.8Co0.15Al0.05]O2 cathode in lithium-ion cells [J]. Power Sources, 2008, 179(1): 347-350.
[10] XIANG J F, CHANG C X, ZHANG F, SUN J T. Rheological phase synthesis and electrochemical properties of Mg-doped LiNi0.8Co0.2O2 cathode materials for lithium-ion battery [J]. Electrochem Soc, 2008, 155(7): 520-525.
[11] WOO S W, MYUNG S T, KIM D W, SUN Y K. Improvement of electrochemical and thermal properties of LiNi0.8Co0.1Mn0.1O2 positive electrode materials by multiple metal (Al, Mg) substitution [J]. Electrochim Acta, 2009, 54(15): 3851-3856.
[12] WANG Xi-min, WANG Xian-you, YI Si-yong, CAO Jun-qi. Synthesis and characteristics of layered LiNi0.8Co0.1Mn0.1O2 cathode material for lithium rechargeable batteries [J]. The Chinese Journal of Process Engineering, 2007, 7(4): 817-821. (in Chinese)
[13] ZHANG Y, CAO H, ZHANG J, XIA B J. Synthesis of LiNi0.6Co0.2Mn0.2O2 cathode material by a carbonate co-precipitation method and its electrochemical characterization [J]. Solid State Ionics, 2006, 177(37/38): 3303-3307.
[14] GUO Hua-jun, LIANG Ru-fu, LI Xin-hai, ZHANG Xin-ming, WANG Zi-xing, PENG Wen-jie, WANG Zhao. Effect of calcination temperature on characteristics of LiNi1/3Co1/3Mn1/3O2 cathode for lithium ion batteries [J]. Trans Nonferrous Met Soc China, 2007, 17(6): 1307-1311.
[15] SCROSATI B. Recent advances in lithium ion battery materials [J]. Electrochim Acta, 2000, 45(15/16): 2461-2466.
_________________________
Foundation item: Project(2007CB613607) supported by National Basic Research Program of China
Corresponding author: LI Xin-hai; Tel: +86-731-88836633; E-mail: csullj@hotmail.com
(Edited by ZHAO Jun)


