
Effects of Na4EDTA and EDTA on seeded precipitation of sodium aluminate solution
L? Bao-lin(吕保林), CHEN Qi-yuan(陈启元), YIN Zhou-lan(尹周澜), HU Hui-ping(胡慧萍)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract:
Na4EDTA and EDTA were adopted as new additives to intensify the seeded precipitation process of sodium aluminate solution. The effects of the two additives at certain concentrations on the seeded precipitation rate of sodium aluminate solution, particle size distribution (PSD) and morphology of precipitated gibbsite were investigated using titration method, particle size analyzer and scanning electron microscope (SEM), respectively. The results show that the two additives can accelerate the seeded precipitation rate of sodium aluminate solution. At relatively high concentration, the facilitative effect of EDTA on sodium aluminate solution is more obvious than that of Na4EDTA. EDTA makes gibbsite particles thinner than Na4EDTA. The Na+ and H+ result in the different effects on the seeded precipitation rate of sodium aluminate solution in spite of the same EDTA anion in the two additives.
Key words:
sodium aluminate solution; seeded precipitation rate; Na4EDTA; EDTA; interaction mechanism;
____________________________________________________________________________________________
1 Introduction
The Bayer process is used for more than a century to extract alumina (Al2O3) from bauxite ores by crystallizing gibbsite (Al(OH)3) from sodium aluminate solution[1]. However, the low ratio of precipitation of gibbsite from the seeded caustic sodium aluminate liquors his a “bottleneck” of the production of alumina for many years[2]. Quite a few of efforts and methods have been devoted to elucidating the precipitation mechanism of sodium aluminate solution for intensifying the seeded precipitation process of sodium aluminate solution. Optimizing the operating conditions, activating the seed and using additives are the mainly intensifying measures that have been employed in the production of gibbsite. Among these methods, using additives as an intensifying method without changing the present equipments and the technological processes, is considered to be one of the best methods. So far, a few works have been mostly focused on organic polymers[3-4] and surfactants as additives[5-8] to intensify the seeded precipitation of sodium aluminate solution. However, how these additives interact with sodium aluminate solution system still requires further investigation.
In this work, Na4EDTA and EDTA with special structure are adopted as additives to promote the precipitation process of sodium aluminate solution. The effect of Na+, H+ and anion of EDTA in the two additives on the precipitation was investigated. This method can offer a meaningful guide for elucidating the seeded precipitation mechanism and intensifying the seeded precipitation process of sodium aluminate solution with other additive.
2 Experimental2.1 Experimental materials and apparatus
Distilled water (single distilled water) was self made. Aluminate hydroxide (industrial grade, supplied by Zhengzhou Research Institute of Aluminum Corporation of China, Limited) was washed and dried. Sodium hydroxide (analytical reagent, Shantou Xilong Chemical Company of China) was adopted in the experiments. Crystal seeds (obtained from Zhengzhou Research Institute of Aluminum Corporation of China Limited) were washed, dried and then passed through a sieve of 45 μm. The minus 45 μm seeds were mixed homogeneously for experiments. Na4EDTA and EDTA (analytical reagent, Shantou Xilong Chemical Company of China) were adopted as additives for the experiments.
Precipitation tank with a volume of 1 L and valid volume of 0.8 L is a stainless steel vessel. The tank was set in an isothermal bath.
2.2 Experimental methods
A certain volume and concentration of sodium aluminate liquors were added into the precipitation tank which was preheated to a constant temperature, then additive was added into the liquor at a constant temperature. The crystal seeds were added and the reaction time was recorded after 20 min isotherm process. The seeded precipitation experiments were performed under the conditions of alkali (Na2Ok) concentration 140 g/L, initial molecular ratio of Na2Ok to Al2O3 (αk) 1.45, temperature (75±0.2) ℃, agitation rate 100 r/min, seed mass ratio (Ks) 0.25, precipitation time 10 h. The samples were taken periodically from precipitation tank and centrifuged. Clear liquors were used to analyze the content of alumina and alkali.
The metallurgical industry standard of YB-817-75 was adopted for the analysis of chemical components. This is a titrimetric method based on the work of Watts[9]. The particle size analyzer of Mastersizer 2000 was used for the PSD determination, and the morphology of gibbsite was observed using a Jsm-6360LV scanning electron microscope.
3 Results and discussion3.1 Effects of Na4EDTA and EDTA at different concentrations on seeded precipitation rate of sodium aluminate solutions
Fig.1 shows the effects of Na4EDTA and EDTA on the seeded precipitation rates of sodium aluminate solutions. It is indicated that the two additives at certain concentrations can all accelerate the seeded precipitation of sodium aluminate solutions. In addition, the similarly facilitative effects of Na4EDTA and EDTA on the seeded precipitation rates of sodium aluminate solutions occur compared with the blank at relatively low concentrations (shown in Figs.1(a) and (b)). However, when the concentrations of the two additives reach 5 mmol/L or 10 mmol/L (shown in Figs.1(c) and (d)), the facilitative effect of EDTA on the seeded precipitation rate of sodium aluminate solution is more obvious than that of Na4EDTA.
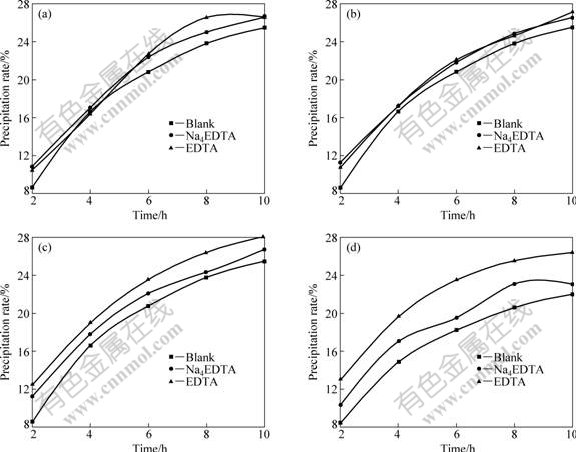
Fig.1 Effect of additive on seeded precipitation rate of aluminate sodium aluminate: (a) 0.1 mmol/L Na4EDTA and 0.1 mmol/L EDTA; (b) 1 mmol/L Na4EDTA and 1 mmol/L EDTA; (c) 5 mmol/L Na4EDTA and 5 mmol/L EDTA; (d) 10 mmol/L Na4EDTA and 10 mmol/L EDTA
3.2 Effects of Na4EDTA and NaCl on seeded precipitation rate of sodium aluminate solution
In order to evaluate the effect of Na+ on the seeded precipitation of sodium aluminate solution, the effects of 10 mmol/L Na4EDTA and 10 mmol/L NaCl on the seeded precipitation rate of sodium aluminate solution were investigated (see Fig.2). It can be seen from Fig.2 that, Na4EDTA promotes the seeded precipitation process of sodium aluminate solution during the precipitation period of 10 h, while NaCl hardly influences firstly and then accelerates gibbsite crystallization.
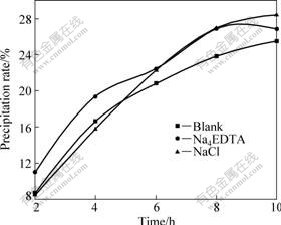
Fig.2 Effect of 10 mmol/L Na4EDTA and NaCl on seeded precipitation rate of sodium aluminate solution
3.3 Effects of Na4EDTA and EDTA on particle size distribution (PSD) of precipitated gibbsite
The effects of 10 mmol/L Na4EDTA and EDTA on the particle size distributions (PSD) of gibbsites Precipitated for 0.5 h are shown in Fig.3. From Fig.3, gibbsite shows an obviously bimodal particle size distribution with an increased contribution to in the range of 0-10 μm in diameter with the addition of 10 mmol/L EDTA into sodium aluminate solution relative to the blank. This phenomenon is not apparent under the same conditions for Na4EDTA. Therefore, it can be concluded that the addition of EDTA leads to the refinement of gibbsite particles newly-precipitated from sodium aluminate solution.

Fig.3 PSD of gibbsite precipitated for 0.5 h
3.4 Typical morphologies of precipitated gibbsite samples
Fig.4 shows the typical SEM images of gibbsite samples precipitated for 0.5 h. It can be seen from Figs.4(a) and (b) that, for precipitated gibbsite samples from the blank sodium aluminate solution and with the addition of 10 mmol/L Na4EDTA, the dispersion of gibbsite particles with diameter under 5 μm on gibbsite surface is not apparent. However, it can be seen from Fig.4(c) that some fine gibbsite particles with diameter under 5 μm disperse on the large gibbsite surface when 10 mmol/L EDTA was added in sodium aluminate solution. The result indicates that the addition of EDTA can lead to the refinement of gibbsite particles precipitated from sodium aluminate solution.
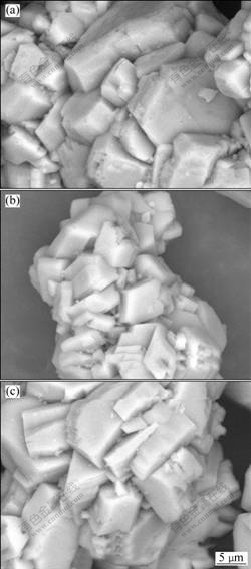
Fig.4 Typical SEM images of gibbsite samples precipitated for 0.5 h: (a) Blank solution; (b) 10 mmol/L Na4EDTA; (c) 10 mmol/L EDTA
3.5 Seeded precipitation mechanism of sodium aluminate solution in Na4EDTA or EDTA
As discussed in section 3.1, the seeded precipitation rate of sodium aluminate solution with the proper concentration of Na4EDTA or EDTA additive increases compared with that of the blank. However, EDTA shows more facilitative effect on the seeded precipitation of sodium aluminate solution than Na4EDTA at relatively high concentration (as shown in Figs.1(c) and (d)).
In simplest form, the ionization equation of NaCl and Na4EDTA expressed by Na4Y (Y refers to anion of EDTA) in sodium aluminate solution may be represented as
NaCl→Na++Cl- (1)
Na4Y→Na++Y4- (2)
As discussed in section 3.2, 10 mmol/L NaCl inhibits firstly and then accelerates the gibbsite crystallization from seeded sodium aluminate solution compared with the blank. According to Eq.(1), the effect of NaCl can be regarded as the combined effect of Na+ and Cl-. YANG[10] investigated the inhibitory effect of Cl- on the seeded precipitation of sodium aluminate solution. The results can deprive from the formation of Cl-—H2O hydrogen bond[11-13], which hinders the migration of free water molecule in the transformation of low coordinated aluminate ion into high coordinated aluminate ion[14]. It can be deduced from Fig.2 that, compared with the blank, Na+ promoting gibbsite crystallization from sodium aluminate solution can be interpreted by three factors as follows:
1) A medium of [Na(H2O)4+·Al(OH)4-] is beneficial to the formation of growth unit[14].
2) A hydrogen bond maker[15] can be beneficial to the intensification of hydrogen bond between aluminate ions to increase the local concentration of aluminate ions.
3) An absorbant of electropositive gibbsite[16] is advantageous to the adsorption of electronegative aluminate ions on the surface of gibbsite.
Therefore, it can be concluded that Na+ can play an important role in the enhancement of the seeded precipitation rate when Na4EDTA is added into sodium aluminate solution.
The structure of EDTA anion is shown in Fig.5.
![]()
Fig.5 Structure of EDTA anion
In previous work, YIN et al[17] pointed out that the facilitative effect of glutamic acid on sodium aluminate solution results from the hydrogen bond action between aluminate ion and glutamic acid. The schematic diagram of the hydrogen bond action between aluminate ion and glutamic acid is shown in Fig.6. According to their opinion, it may be assumed that the hydrogen bond action between aluminate ion and anion of EDTA can occur according to the structure of anion of EDTA. Therefore, it may be concluded that anion of EDTA is another important factor which leads to the increase of the precipitation rate of sodium aluminate solution.

Fig.6 Schematic diagram of hydrogen bond action between aluminate ion and glutamic acid[17]
The behavior of EDTA represented by H4Y (Y refers to anion of EDTA) in sodium aluminate solution can be phenomenologically divided into two steps. In simplest form, the first step is the ionization of EDTA in sodium aluminate solution, which can be expressed as
H4Y→4H++Y4- (3)
Eq.(3) suggests that the appearance of anion of EDTA in sodium aluminate solution should promote the seeded precipitation process of sodium aluminate solution compared with the blank according to the result analyzed above.
In simplest form, the second step is the reaction between H+ and Al(OH)4- in sodium aluminate solution, which can be expressed as
H++Al(OH)4-→Al(OH)3+H2O (4)
However, sodium aluminate solution includes other active aluminate ions, such as dimers, cyclic hexamers, sodium ion pairs or polyaluminate clusters of unknown and probably variable stoichiometry besides Al(OH)4-[18], the only certainties in respect of aluminate ions speciation in supersaturated solutions are that at least one other polyaluminate ion exists in equilibrium with the Al(OH)4-. Eq.(4) shows that the acidity of EDTA is beneficial to the formation of Al(OH)3.
XIE et al[19] reported that a proper concentration of aluminum salt such as AlF3, AlCl3, Al2SO4 and K2SO4·Al2SO4·24H2O can lead to the formation of aluminum colloid due to hydrolysis. The aluminum colloid becomes the crystallization centre. The new crystallization centre is highly active, thereby accelerates the seeded precipitation of sodium aluminate solution, but results in the refinement of gibbsite particles. According to this idea, it may be assumed that the newly formed Al(OH)3 described by Eq.(4) as a form of crystallization center of aluminum colloid accelerates the seeded precipitation process of sodium aluminate solution and leads to the refinement of gibbsite particles (shown in Figs.3 and 4(c)) with the addition of EDTA into sodium aluminate solution.
In summary, for Na4EDTA, the increase of the precipitation rate of sodium aluminate solution could result from the combined actions of Na+ and EDTA anion in sodium aluminate solution, and for EDTA, the increase of the seeded precipitation rate of sodium aluminate solution could originate from the combined effects of H+ and EDTA anion in sodium aluminate solution.
4 Conclusions1) Na4EDTA and EDTA show facilitative effects on the precipitation of sodium aluminate solutions. At relatively high concentrations (5 mmol/L and 10 mmol/L), the facilitative effect of EDTA is more obvious than that of Na4EDTA.
2) Adding EDTA into sodium aluminate solution leads to the refinement of gibbsite particles and the dispersion of small gibbsite particles on large gibbsite surface compared with the blank and the sodium aluminate solution with the addition of Na4EDTA.
3) The facilitative effect of Na4EDTA on sodium aluminate solution could result from the combined actions of Na+ and EDTA anion in sodium aluminate solution. Adding EDTA into the sodium aluminate solution could result in the combined effects of H+ and EDTA anion in sodium aluminate solution.
References[1] ISABELLE S, ST?PHANE V, G?RARD P, ROLAND B. The influence of additives on the crystal habit of gibbsite [J]. Journal of Crystal Growth, 1999, 196(1): 174-180.
[2] ZENG Ji-shu, YIN Zhou-lan, CHEN Qi-yuan. Intensification of precipitation of gibbsite from seeded caustic sodium aluminate liquor by seed activation and addition of crown ether [J]. Hydrometallurgy, 2007, 89(1/2): 107-116.
[3] ROE W J, PERISHO J L. Use of polymers in alumina precipitation in the Bayer process of bauxite beneficiation: US4608237 [P]. 1986-08-26.
[4] YIN Zhou-lan, JING Ye-ling, CHEN Qi-yuan, ZHANG Ai-min. Effect of polymers on seed precipitation of sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2007, 17(6): 1002-1007. (in Chinese)
[5] OWEN D O, DAVIS D C. Use of surfactants in alumina precipitation in the Bayer process: US4737352 [P]. 1988-10-12.
[6] ZHAO Su, BI Shi-wen, DING Xiang-qun, TONG Zhi-fang. Influence of novel surfactants on the physicochemical properties of sodium aluminate solution relevant to the aluminium hydroxide precipitation process [J]. Mineral Processing and Extractive Metallurgy, 2005, 114(4): 53-56.
[7] MAHONEY R P, SCHNIEDERS JR W B. Trihydrate crystal modifier in the Bayer process: US0159936 [P]. 2002-10-31.
[8] ZENG Ji-shu, YIN Zhou-lan, CHEN Qi-yuan. Effect of tetracarbon additives on gibbsite precipitation from seeded sodium aluminate liquor [J]. J Cent South Univ Techno, 2008, 15(5): 622-626.
[9] WATTS H L, UTLEY D W. Volumetric analysis of sodium aluminate solutions [J]. Analytical Chemistry, 1953, 25(6): 864-867.
[10] YANG Chong-yu. Production technology of alumina [M]. Beijing: Metallurgical Industry Press, 1993: 132-134. (in Chinese).
[11] KROPMAN M F, BAKKER H J. Dynamics of water molecules in aqueous solvation shells [J]. Science, 2001, 291(5511): 2118-2120.
[12] RULL F. Structural investigation of water and aqueous solutions by raman spectroscopy [J]. Pure Appl Chem, 2002, 74(10): 1859-1870.
[13] LI Rui-hua, JIANG Zhan-peng, CHEN Fen-gen, YANG Hong-wei, Guan Yun-tao. Hydrogen bonded structure of water and aqueous solutions of sodium halide: a Raman spectroscopic study [J]. Journal of Molecular Structure, 2004, 707(1/3): 83-88.
[14] LI Jie. Study on the structural characteristics and decomposition mechanism of supersaturated sodium aluminate solution [D]. Changsha: Central South University, 2001: 88-91. (in Chinese)
[15] LI Rui-hua, JIANG Zhan-peng, SHI Shao-qi, YANG Hong-wei, YUAN Shu-yu. Effect of common ions in water on water association by 17O-NMR [J]. Environmental Science, 2002, 23(3): 44-48.(in Chinese)
[16] WILLIAM N R, RICHARD W O, ROBERT J H. Surface properties of aluminum hydroxide at high salt concentration [J]. Journal of Colloid and Interface Science, 1997, 188(2): 325-335.
[17] YIN Zhou-lan, MA Ying, LV Bao-lin, LI Jia-ping, CHEN Qi-yuan. Effects of glutamic acid additives on seeded precipitation of sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(s1): s130-s133. (in Chinese)
[18] WATLING H. Gibbsite crystallization inhibition (2). Comparative effects of selected alditols and hydroxycarboxylic acids [J]. Hydrometallurgy, 2000, 55(3): 289-309.
[19] XIE Yan-li, BI Shi-wen, REN Wen-cai. Application of additives in precipitation of Bayer sodium aluminum solution [J]. Light Metals, 2000(1): 25-26, 49. (in Chinese)
__________________________________
Foundation item: Project(20476107) supported by the National Natural Science Foundation of China; Project(2005CB623702) supported by the National Basic Research Program of China
Corresponding author: YIN Zhou-lan; Tel: +86-731-88877364; E-mail: lbl7701951@163.com


