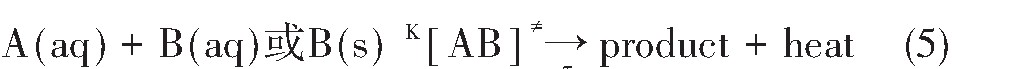网络首发时间: 2019-11-13 17:24
微量热法研究白钨矿在NaOH溶液中的溶解及其与油酸钠作用的热动力学
江西理工大学资源与环境工程学院
摘 要:
采用微量热法研究了白钨矿在NaOH溶液中的溶解及其与油酸钠作用的热动力学,并通过扫描电子显微镜(SEM)、总有机碳(TOC)及傅里叶变换红外光谱(FTIR)对白钨矿在NaOH溶液体系中的溶解特性及其与油酸钠的作用方式进行表征,同时进行了单矿物浮选试验研究。结果表明:白钨矿在NaOH溶液中溶解为吸热反应,反应级数n=3,pH=10时溶解反应热最大,溶解反应热与Ca2+浓度成正相关。白钨矿表面Ca2+在NaOH溶液中吸附油酸根离子为放热反应,反应级数n=1,相对白钨矿溶解发生了热量转变,不同pH条件下的吸附反应热大小与白钨矿溶解反应热趋势一致,在pH=12时反应速率均较快。不同pH条件下油酸根离子的吸附量与其反应热大小成正比,这与单矿物浮选结果一致。
关键词:
中图分类号: TD954;TD923
作者简介:匡敬忠(1971-),男,江西泰和人,博士,教授,研究方向:矿物分选理论与工艺、矿物材料及二次资源综合利用,电话:0797-8123759,E-mail:kjz692@163.com;
收稿日期:2019-05-17
基金:国家自然科学基金项目(51664019)资助;
Dissolution of Scheelite in NaOH Solution and its Interaction with Sodium Oleate by Microcalorimetry
Kuang Jingzhong Ma Qiang Liu Pengfei Huang Zheyu
Faculty of Resource and Environmental Engineering,Jiangxi University of Science and Technology
Abstract:
Microcalorimetry was an on-line and non-destructive method for the determination of thermal effects. The change of enthalpy and specific heat caused by the reaction of material was measured and analyzed by microcalorimeter,and the corresponding thermodynamic and kinetic parameters were obtained to study the reaction process. At present,there were few reports on the dissolution and adsorption of scheelite under alkaline conditions by microthermal method. Therefore,the study of the thermodynamic and kinetic parameters of the dissolution and surface adsorption of scheelite under alkaline conditions by microthermal method had important theoretical guiding significance. The scheelite used in this study were a synthetic one,and the Na2WO4 and Ca(NO3)2 used in the synthesis of scheelite were both analytically pure. The pH regulators were NaOH and its purity was analyzed. The collector was sodium oleate,analytically pure. The experimental water was ultrapure water. The trace thermal kinetics of scheelite dissolution and scheelite adsorption sodium oleate were tested by Microcalorimeter. The absorption quantity of sodium oleate on scheelite surface was tested by adsorption experiment. The structure transformation of scheelite surface was tested by Fourier infrared spectrum analysis and verified by flotation experiment. The results mainly included the following five parts:(1)It could be seen that the dissolution of scheelite in NaOH solution was endothermic reaction. At pH=10,the integral area of the heat rate curve(heat of reaction)was at its maximum Q=-24.38 mJ,and at pH=12,the minimum heat of reaction Q=-18.99 mJ. The order of the reaction heat dissolved in NaOH solution of scheelite was pH=10>pH=11>pH=9>pH=8>pH=12. In short,the reaction rate constant(k)tended to decrease first and then increase. When pH=12,the reaction rate constant reached its maximum. The reaction order(n)was approximately equal to 3,indicating that scheelite wasdissolved in NaOH at different pH values as a third-order reaction.(2)D50 changed with pH after dissolution of scheelite in NaOH solu-tion,and the result was pH=10>pH=11>pH=9>pH=8>pH=12,which was consistent with the law of Ca2+dissolution. The reaction heat of dissolution was positively correlated with the concentration of Ca2+.(3)The reaction of scheelite with sodium oleate in NaOH was exothermic. The order of reaction heat of scheelite surface acting with sodium oleate was pH=10>pH=11>pH=9>pH=8>pH=12. There was a faster reaction rate in low base and high base. The reaction order(n)was approximately equal to 1,indicating that scheelite reacts with sodium oleate in NaOH with different pH values as a first-order reaction.(4)According to the Fourier infrared spectrum,1546 cm-1 was the asymmetric stretching vibration absorption peak of-COO-,the carboxylic acid negative ion(-COO-)in the calcium oleate molecule produced anti-symmetric stretching vibration,and was the characteristic spectral band of calcium oleate. It could be seen that the oleate ion was adsorbed on the scheelite surface to produce calcium oleate,which was chemical adsorption. The peak strength of anti-symmetric stretching vibration generated from carboxylic acid radical anion(-COO-)to small was pH=10>pH=11>pH=9>pH=8>pH=12. This was consistent with the pH sequence of sodium oleate adsorption.(5)According to the flotation experiment,when the natural pH was 7.3,the concentration of sodium oleate had a great impact on the flotation recovery of scheelite. With the increase of the concentration of sodium oleate,the flotation recovery of scheelite first increased and then decreased,and reached a maximum of 81.72% when the concentration of sodium oleate was 40 mg·L-1. The optimal concentration of sodium oleate was selected to be 40 mg·L-1. With the increase of pH,the flotation recovery of scheelite first increased. When pH=10,recovery had maximum,and the pH continued to increase,the flotation recovery of scheelite began to decline. The order of the recovery rate of scheelite flotation was pH=10>pH=11>pH=9>pH=8>pH=12. It was clear that the Ca2+on the surface of tungsten ore would be adsorbed with oleic acid ions by chemically,and the formation of calcium oleate would lead to the enhancement of its hydrophobicity,so as to achieve the purpose of flotation scheelite. Scheelite dissolved in NaOH solution as endothermic reaction,reaction order n=3. When pH=10,the maximum heat of dissolution Q was-24.38 mJ;when pH=12,the minimum heat of dissolution Q was-18.99 mJ;the order of dissolution heat was pH=10>pH=11>pH=9>pH=8>pH=12;the dissolution heat was positively correlated with the concentration of Ca2+,and the dissolution rate was the fastest when pH=12. Adsorption of scheelite in NaOH solution to sodium oleate was an exothermic reaction,with reaction order n=1.Relative to scheelite dissolution,there was a heat change,which was a spontaneous reaction. The order of reaction heat of adsorption sodium oleate by scheelite was consistent with that of dissolution reaction heat of scheelite. When pH=10,the maximum heat of adsorption reaction Q was 18.77 mJ;when pH=12,the minimum heat of adsorption reaction Q was 16.60 mJ;when pH=12,the adsorption reaction rate was the highest. Under different pH conditions,the adsorption quantity of oleic acid ions was in direct proportion to its reaction heat.The infrared spectrum test results showed that oleic acid ions were adsorbed on the scheelite surface and Ca2+generate calcium oleate,resulting in its enhanced hydrophobicity. Moreover,the absorption peak strength and adsorption quantity were in the same order,which was consistent with the flotation results.
Keyword:
microcalorimetry; thermokinetics; scheelite; sodium oleate; dissolution; adsorption;
Received: 2019-05-17
微量热法是近年来发展起来的一种在线、无损伤的热效应测定方法。采用微量热仪对物质发生反应时所引起的热焓及比热变化进行测定分析,并获得相应的热力学、动力学参数可研究其反应过程
界面反应是指两相之间接触表面上由于化学物质种类、存在状态及含量等性质的不同发生的化学反应。众所周知,药剂在矿物表面的吸附形式分为物理吸附和化学吸附两种形式。利用微热量计测量热效应并计算得到的热动力学参数可用来研究矿物表面吸附药剂的热动力学变化规律,推测吸附机制
白钨矿与含钙脉石矿物具有相似的晶体结构,破碎时解离面都会沿着金属离子占优势的面产生,同时在矿物表面暴露出大量高能态Ca2+,使得白钨矿与含钙脉石矿物有相近的表面理化特征。同时白钨矿表面吸附药剂时伴随着矿物溶解,溶解组分与白钨矿表面作用会导致矿物表面物理化学性质、甚至浮选行为的改变,造成白钨矿浮选困难
本文以合成白钨矿为研究对象,采用微量热法研究了白钨矿在Na OH溶液中的溶解及其与油酸钠作用的热动力学。通过扫描电子显微镜(SEM)、总有机碳(TOC)及傅里叶变换红外光谱(FTIR)分析了白钨矿在Na OH溶液体系中的溶解特性及其对油酸钠的吸附机制,并通过单矿物浮选试验进行验证,为白钨矿在碱性体系下的浮选提供理论基础。
1实验
1.1原料制备及试剂
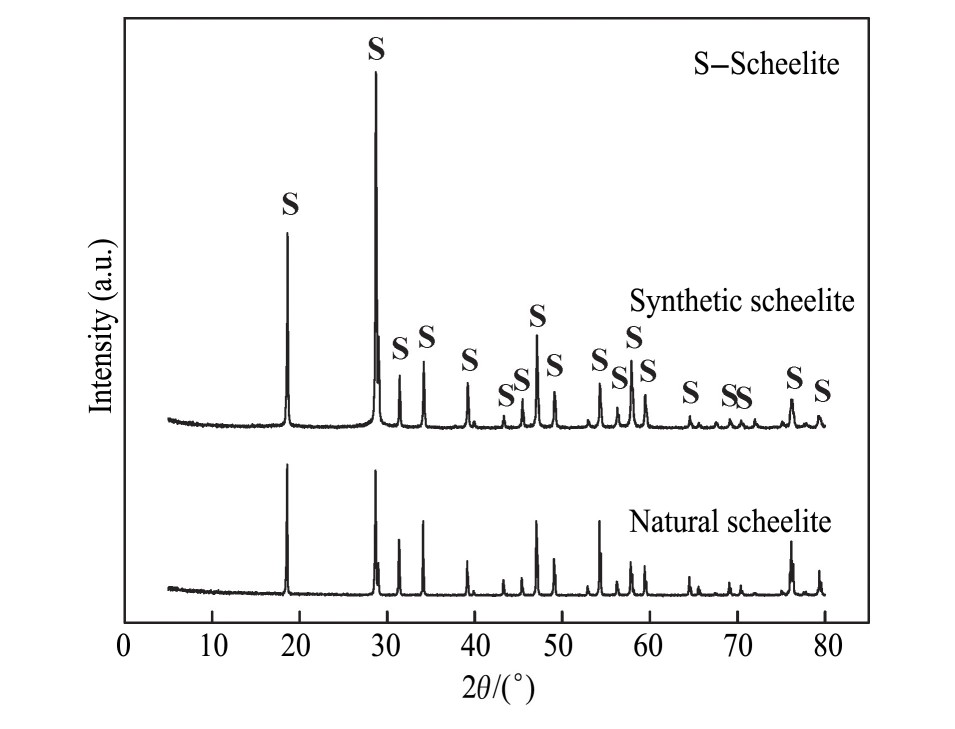
本研究所用的白钨矿为人工合成矿,合成方法:配制0.2 mol·L-1的Na2WO4和Ca(NO3)2溶液,控制溶液流速为3.6 ml·min-1,混合溶液在超声波功率为900 W条件下超声处理5 min。磁力搅拌2 h,陈化,过滤,洗涤,干燥,得到人工合成白钨矿。合成白钨矿与天然白钨矿的X射线衍射(XRD)图谱见图1。由图1可知,合成白钨矿与天然白钨矿衍射峰位一致,化验结果显示合成白钨矿纯度为97.75%。合成白钨矿激光粒度分布结果见图2,由图2可知,合成白钨矿中位径(D50)=15.90μm,合成白钨矿粒度较细,尺寸均匀,适用于研究白钨矿溶解及与油酸钠吸附过程。合成白钨矿所用的Na2WO4和Ca(NO3)2均为分析纯;p H调整剂为Na OH,分析纯;捕收剂为油酸钠,分析纯。实验用水为超纯水。
1.2试验方法
1.2.1微量热试验
(1)白钨矿溶解微量热试验:称取白钨矿0.02 g,置于测试池玻璃外管中,并往玻璃内管中分别加入1.2 ml p H为8,9,10,11,12的Na OH溶液。将内管放入外管中,再将外管置于不锈钢套筒内,放入测量池待测。参考池中玻璃外管不加入白钨矿,只在内管中加入等量的p H分别为8,9,10,11和12的Na OH溶液。设定温度参数为298.15 K,待基线平稳,同时捅破玻璃内管,测量其溶解热动力学参数,每次试验重复3次取平均值。
图1 合成白钨矿及天然白钨矿XRD图谱
Fig.1 XRD patterns of synthetic scheelite and natural scheelite
图2 合成白钨矿的粒度分布曲线
Fig.2 Grain size distribution curve of synthetic scheelite
(2)白钨矿吸附油酸钠微量热试验:将0.5 g白钨矿分别溶解在50 ml p H为8,9,10,11,12的Na OH溶液中,取其溶液2 ml置于玻璃外管中,往内管中加入1.0 g·L-1的油酸钠溶液1.2 ml。然后将内管放入外管中,再将外管置于不锈钢套筒内,放入测量池待测。参考池玻璃外管不加白钨矿,只在内管加入等量油酸钠溶液。设定温度参数为298.15K,待基线平稳,同时捅破玻璃内管,测量其吸附热动力学参数,每次试验重复3次取平均值。
1.2.2溶解及吸附平衡时间试验
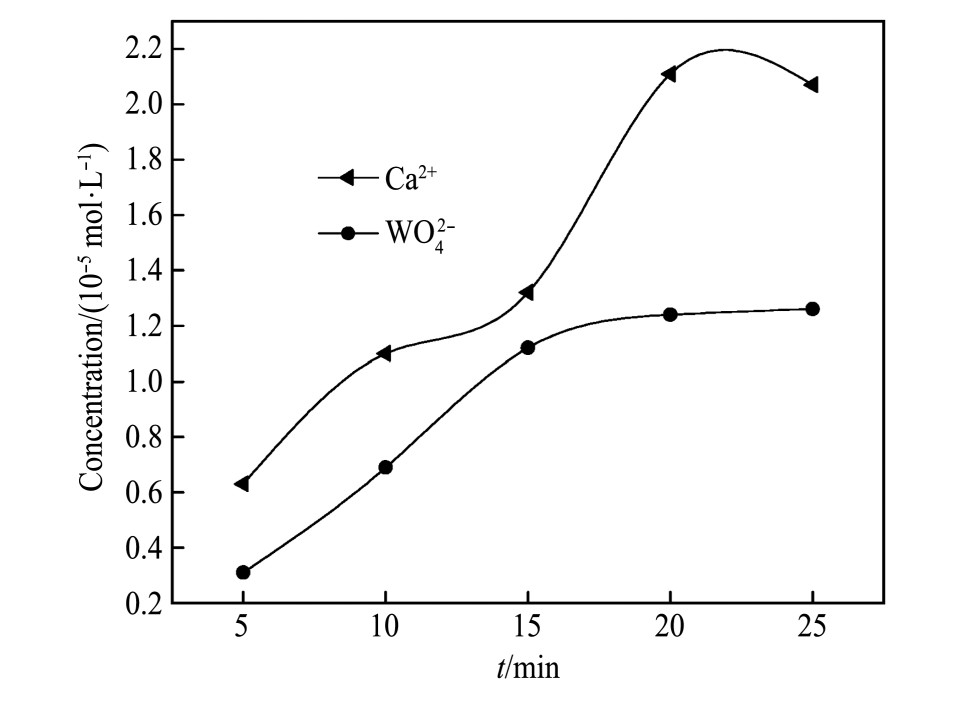
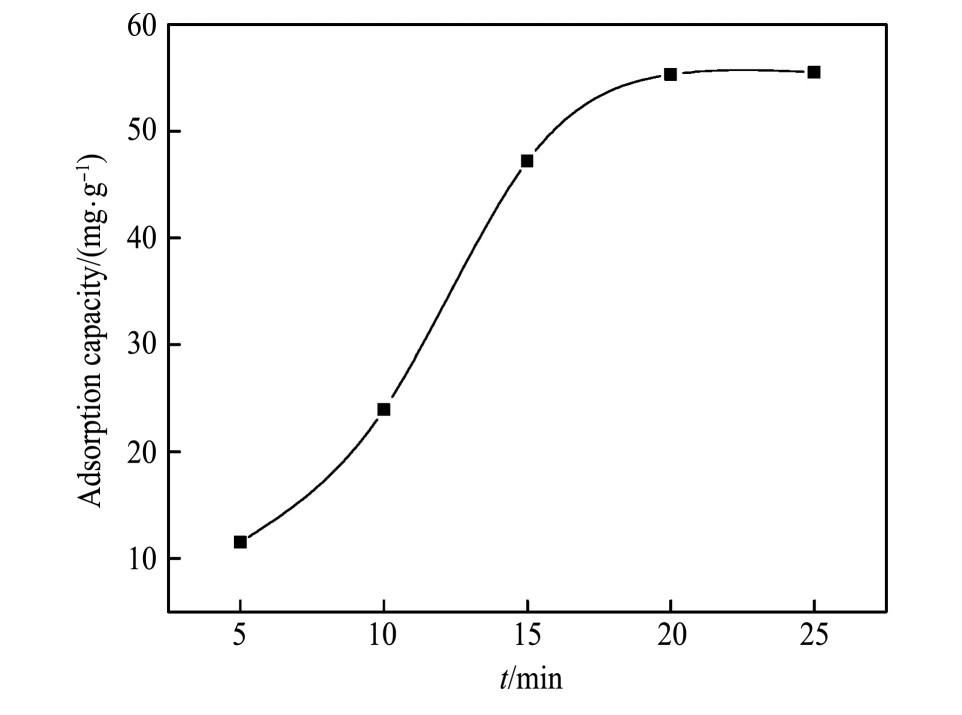
将1.0 g白钨矿加入100 ml不同p H溶液中,磁力搅拌20 min,然后8000 r·min-1离心机离心5 min,取出上清液,测量Ca2+,WO42-浓度。Ca2+,WO42-浓度随反应时间变化结果如图3,由图3可知,随着时间的增加,Ca2+,WO42-浓度升高,在20 min时达到溶解平衡。称取1.0 g白钨矿置于100 ml烧杯中,加入1 g·L-1的油酸钠溶液60 ml,加入超纯水约至100 ml,磁力搅拌20 min,然后8000 r·min-1离心机离心5min,取出上清液待测。以式(1)计算油酸钠吸附量。油酸钠吸附量随反应时间变化结果如图4,由图4可知,随着时间的增加,油酸钠吸附量升高,在20 min时达到吸附平衡。因此,确定溶解及吸附平衡试验固定反应时间为20 min。
图3 Ca2+,WO42-浓度随反应时间变化结果
Fig.3 Changes of Ca2+,WO42-concentration with reaction time
图4 油酸钠吸附量随反应时间变化结果
Fig.4 Results of adsorption of sodium oleate with reaction time
式中,τ为油酸钠吸附量,mg·g-1;c0为原始油酸钠浓度,mg·L-1;ce为上清液油酸钠浓度,mg·L-1;V为上清液体积,L;m为白钨矿质量,g。
1.2.3溶解试验
用Na OH溶液(400 mg·L-1)调节制备p H为8,9,10,11,12的溶液,将1.0 g白钨矿加入100 ml不同p H的溶液中,磁力搅拌20min,然后8000 r·min-1离心机离心5 min,取出上清液,过滤,测量Ca2+,WO42-含量。其中WO42-含量测量参考国标(GB/T 14352.1—2010)。在盐酸介质中,Ti Cl3可将WO3,WO42-还原至+5价并与硫氰酸钾形成黄色络合物,与分光光度计上在λ=420 nm处测量吸光度。原理如式(2),(3)
根据标准液吸光度数据进行线性拟合得到WO3测试标准曲线见图5。
拟合后可得方程:Abs.=0.01274 c+0.00657,线性相关性系数R2=0.9988。其中Abs.表示吸光度,c表示WO3浓度(mg·L-1)。测试溶解样品的吸光度,同等摩尔浓度的WO3,WO42-溶液形成黄色络合物对应吸光度大小相等。对照WO3测试标准曲线计算试样中WO42-的摩尔浓度。
1.2.4吸附量试验
称取1.0 g白钨矿置于100ml烧杯中,加入1 g·L-1的油酸钠溶液60 ml,加入超纯水约至100 ml,用Na OH溶液(400 mg·L-1)分别调节p H为8,9,10,11,12,再滴加少量超纯水补至100 ml。磁力搅拌20 min,然后8000 r·min-1离心机离心5 min,取出上清液待测。以式(1)计算油酸钠吸附量。
1.2.5单矿物浮选试验
试验采用容积为40 ml的XFG型挂槽式浮选机,每次称取2.0 g白钨矿置于浮选槽中,加入适量超纯水搅拌1 min后加Na OH溶液(400 mg·L-1)调节p H并搅拌2 min,加入油酸钠搅拌3 min,浮选3 min。将所得泡沫产品与槽内产品分别烘干、称量,以式(4)计算回收率。
图5 WO3测试标准曲线
Fig.5 Test standard curve of WO3
式中,R为回收率;m1,m2分别为泡沫、槽内产物质量。
1.3测试及表征
采用丹东百特BT-9300ST型全自动激光粒度分析仪测量合成白钨矿的粒度分布。采用丹东方圆的DX-2700型X射线衍射仪对合成的白钨矿进行物相组成分析。测试条件为:工作电压35 k V,工作电流25 m A,扫描步宽为0.02,Cu靶Kα射线。通过绵阳中物热公司RD496-2000型微量热仪测试白钨矿溶解及与油酸钠作用的热动力学参数,测试精度为0.01。采用美国PE公司Optima8000型电感耦合等离子体原子发射光谱仪(ICP)测定Ca2+含量。采用美国PE公司Lambada35型紫外-可见光分光光度计测量WO42-吸光度。采用德国Elementar vario总有机碳(TOC)分析仪对白钨矿表面吸附油酸钠后的上清液进行C元素浓度测定,测试精度为0.001。通过美国FEI公司MLA650F型扫描电镜观察白钨矿溶解前后微观形貌变化。通过Perkin Elmer公司Spectrum Two傅立叶变换型红外光谱分析仪进行油酸钠与白钨矿作用后表面产物结构转变分析,采用KBr压片法测试。每次称取1 mg待测样品,按比例1∶100加入KBr 100 mg研磨待测,控制压力为5 MPa进行压片。
1.4微量热动力学数据处理方法
对等温等压下的任一不可逆反应
和边界条件
当t=0时,C0=1,α0=0,H0=0;
式中,C0和C∞分别为反应开始和反应结束时反应物的分数;α0和α∞分别反应开始和反应结束时已反应物的分数;H0和H∞分别为反应开始和反应结束时反应体系的放热量。
由反应边界条件知反应进度与反应体系能量变化的关系:
由(6)式可知
当t趋向于∞时,α∞=1,则
将式(8)和(9)带入n级微分速率方程
得
采用最小二乘法回归反应的热动力学数据,即可求出反应速率常数K(截距)和反应级数n(斜率)。
2结果与讨论
2.1白钨矿在Na OH溶液中的溶解热动力学
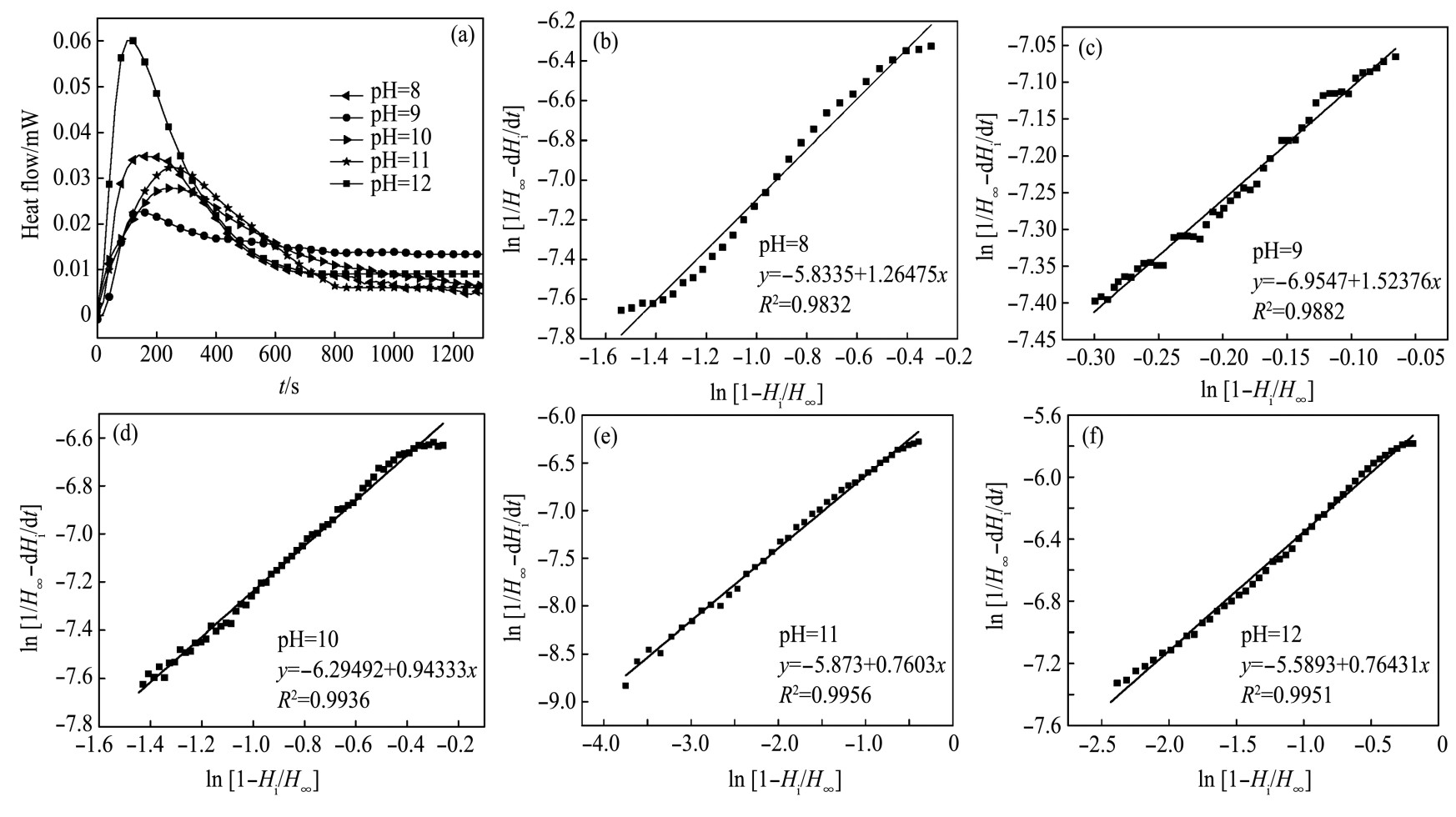
图6(a)为白钨矿在Na OH溶液中溶解的热速率曲线。由图6(a)可知,在p H分别为8,9,10,11,12时对应的溶解热速率曲线均为负,可知白钨矿在Na OH溶液中的溶解为吸热反应。将不同p H溶解微量热数据利用式(12)拟合得图6(b-f),再将拟合数据由式(12)分别计算得表1。表1为白钨矿在Na OH中溶解热动力学参数。由表1可知,p H=10时热速率曲线积分面积(反应热)最大Q=-24.38 m J,p H=12时反应热最小Q=-18.99 m J。白钨矿在Na OH溶液中溶解的反应热大小顺序为(p H=10)>(p H=11)>(p H=9)>(p H=8)>(p H=12)。反应速率常数(K)整体有先降低后增加的趋势,在p H=12时反应速率常数最大。反应级数(n)大致等于3,表明白钨矿在不同p H值的Na OH中溶解为三级反应。
2.2白钨矿在Na OH溶液中的溶解特性
白钨矿在Na OH溶液中存在式(13)~(16)的平衡反应:
图6 白钨矿在Na OH中的溶解热速率曲线及数据拟合图
Fig.6 Heat rate curve(a)and data fitting graph(b-f)of scheelite dissolution in Na OH solution
表1 白钨矿在Na OH溶液中溶解热动力学参数 下载原图
Table 1 Thermodynamic parameters of scheelite dissolved in Na OH solution
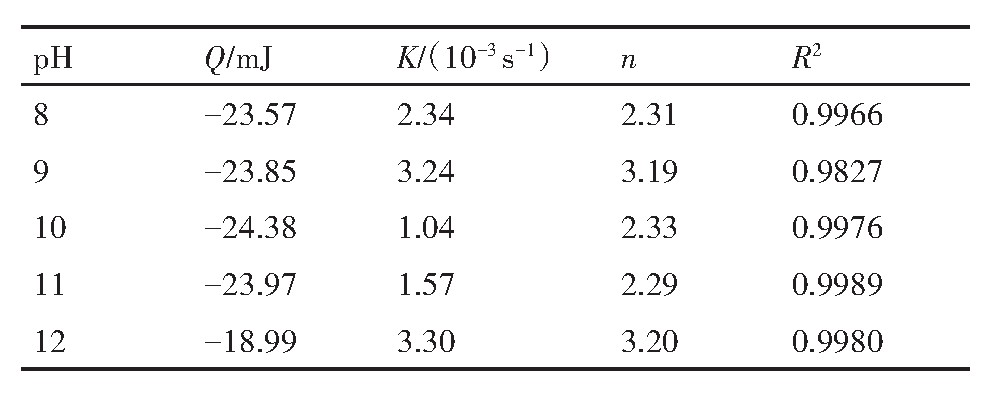
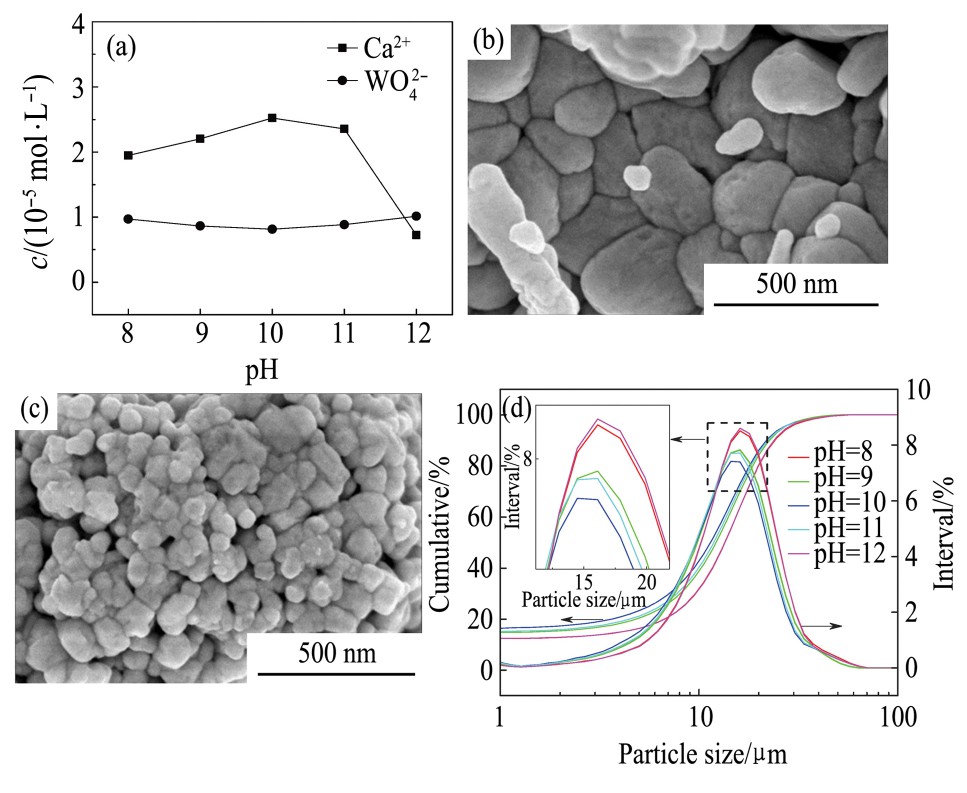
图7(a)为白钨矿在Na OH溶液中的溶解特性图。由图7(a)可知,体系中Ca2+浓度先增加后减少,在p H=10时达到最大值2.53×10-5mol·L-1,WO42-浓度先少量降低后增大,在p H=12时达到最大值1.01×10-5mol·L-1。在p H<11.8时,体系中Ca2+浓度大于WO42-浓度,这是由于Ca2+优先溶解导致。随着p H增大,反应(14),(15)逐渐增强,Ca2+浓度逐渐降低。但在p H<10时,随p H增大Ca2+浓度增大,是由于反应(13)反应速率大于反应(14),(15)造成。在p H>11.8时,WO42-浓度大于Ca2+浓度,这是由于在高碱体系发生了反应(16),造成WO42-浓增大。Ca2+浓度由大到小依次为p H=10>p H=11>p H=9>p H=8>p H=12。由图7(a)可知,p H=10时Ca2+浓度达到最大值。故选取在此p H值溶解后的白钨矿与白钨矿原矿进行微观形貌分析。图7(b,c)分别为白钨矿原矿、在Na OH溶液p H=10时白钨矿溶解后白钨矿表观形貌图。由图7(b,c)可知,白钨矿原矿晶体粒径较大并成块状,且白钨矿颗粒间有明显的界限。在p H=10的Na OH溶液中溶解后,白钨矿表面开裂,颗粒出现细化。图7(d)为白钨矿在Na OH溶液中溶解后的粒度分布曲线。由图7(d)可知,白钨矿在p H为8,9,10,11,12的Na OH溶液中溶解时较白钨矿原矿的D50值(15.90μm)减小。表2为白钨矿在Na OH溶液中溶解后D50随p H变化结果,由表2可知,白钨矿在Na OH溶液中溶解后D50随p H变化结果为p H=10>p H=11>p H=9>p H=8>p H=12,这与Ca2+溶解规律一致。结合白钨矿溶解特性分析是由于白钨矿晶体发生式(13)~(16)的平衡反应导致。结合2.1节知,溶解反应热与Ca2+浓度成正相关。
图7 白钨矿在Na OH溶液中的溶解特性、溶解前后的表面微观结构及溶解后的粒度分布曲线
Fig.7 Dissolution characteristics of scheelite in Na OH solu-tion(a),surface microstructure before(b)and after dissolution in Na OH solution with p H=10(c)and grain size distribution curve after dissolution(d)
2.3白钨矿与油酸钠作用热动力学
图8(a)为白钨矿与油酸钠作用热速率曲线。由图8(a)可知,在p H值分别为8,9,10,11,12时对应的热速率曲线均为正,可知在Na OH中白钨矿与油酸钠作用为放热反应。将不同p H下白钨矿与油酸钠作用微量热数据利用式(12)拟合得图8(bf),再将拟合数据由式(12)分别计算得表3。表3为白钨矿与油酸钠作用的热动力学参数。由表3可知,p H=10时热速率反应热最大Q=18.77 m J,p H=12时反应热最小Q=16.60 m J。白钨矿表面与油酸钠作用的反应热大小顺序为p H=10>p H=11>p H=9>p H=8>p H=12。反应速率常数(K)先降低后增加,说明在低碱和高碱中有着较快的反应速率,反应级数(n)大致等于1,表明白钨矿在不同p H值的Na OH中与油酸钠作用为一级反应。结合2.1节可知,白钨矿表面与油酸钠作用相对白钨矿溶解发生了热量转变。对比白钨矿溶解微量热及与油酸钠作用微量热反应速率常数(K)可知,在p H=12时反应速率常数均最大。
表2 白钨矿在不同p H值溶液中溶解后的中位径(D50)值 下载原图
Table 2 Median diameter(D50)of scheherite dissolved in solutions with different p H values

2.4白钨矿与油酸钠作用机制探讨
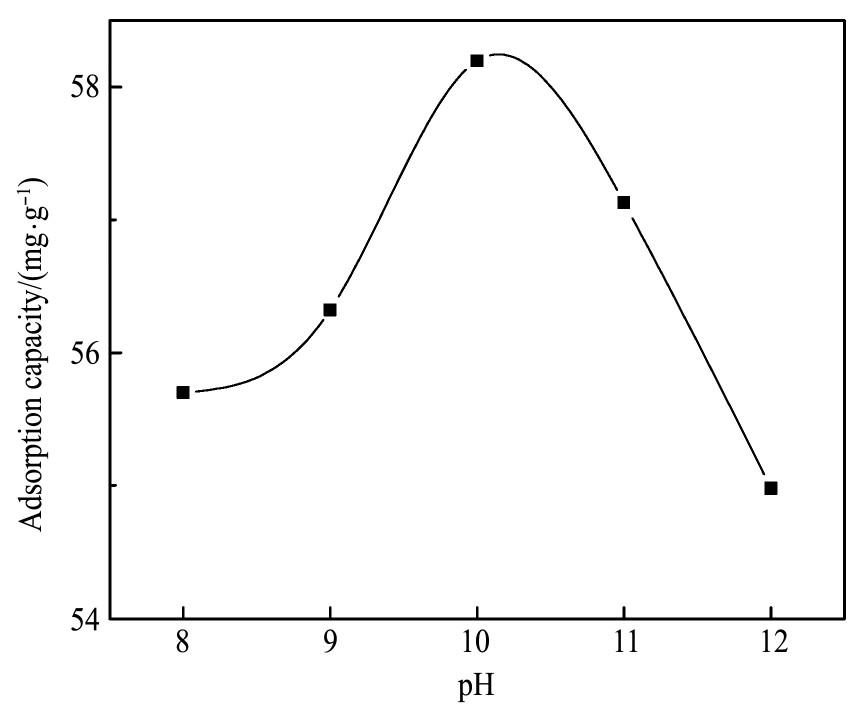
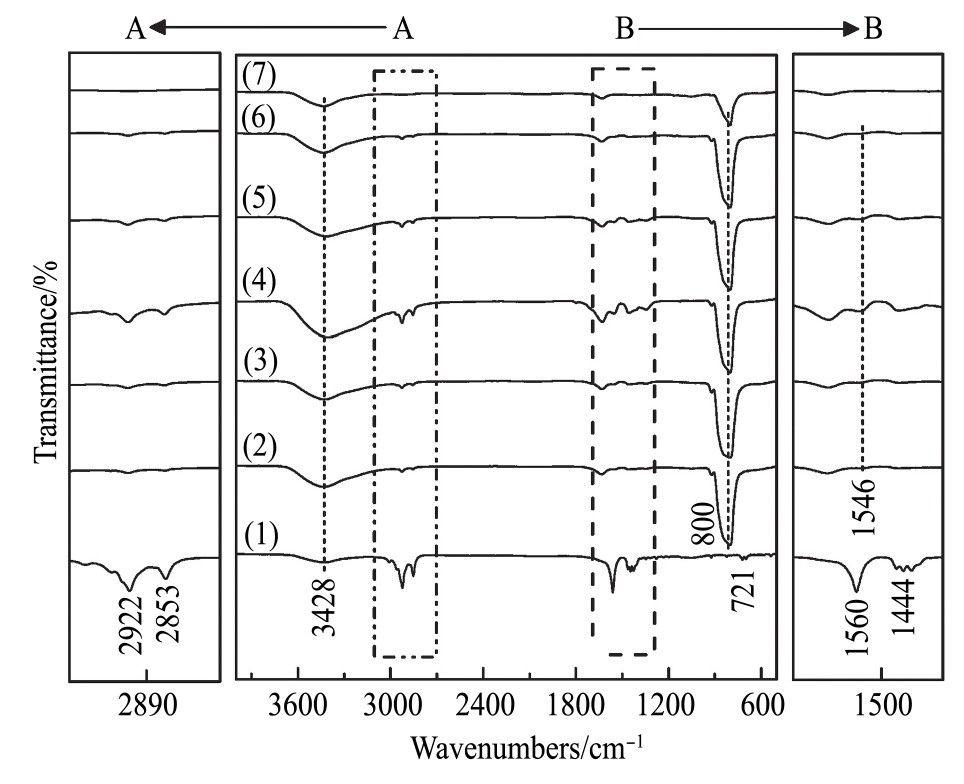
图9为采用剩余浓度法计算得到的油酸钠吸附量曲线。由图9可知,p H=10时,白钨矿表面吸附油酸钠的量最多为58.20 mg·g-1,p H=12时吸附油酸钠的量最少为54.98 mg·g-1。白钨矿吸附油酸钠的量对应的p H顺序为p H=10>p H=11>p H=9>p H=8>p H=12。图10为白钨矿与油酸钠作用红外谱图。由图10可知,由于WO42-的W-O键不对称伸缩振动ν3主要发生在786~883 cm-1之间。对照白钨矿谱图,800 cm-1为钨酸根离子的W-O键不对称伸缩振动吸收峰。3428 cm-1为水的羟基振动峰
图8 白钨矿与油酸钠的热速率曲线及数据拟合图
Fig.8 Heat rate curve(a)and data fitting graph(b-f)of scheelite and sodium oleate
表3 白钨矿与油酸钠作用热动力学参数 下载原图
Table 3 Thermodynamic parameters of interaction be-tween scheelite and sodium oleate
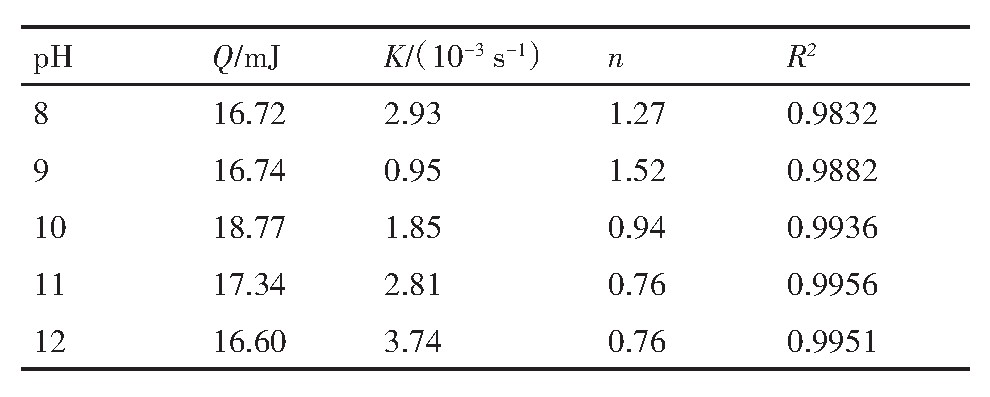
图9 油酸钠吸附量曲线
Fig.9 Curve of sodium oleate adsorption capacity
图1 0 白钨矿与油酸钠作用红外图谱
Fig.10 Infrared spectrum of scheelite interacting with sodium oleate
(1)Sodium oleate;(2)pH=8;(3)pH=9;(4)pH=10;(5)pH=11;(6)pH=12;(7)Scheelite
2.5白钨矿浮选试验
图11为油酸钠浓度对白钨矿浮选回收率的影响结果。由图11可知,在自然p H=7.3时油酸钠的浓度对白钨矿浮选回收率有很大影响,随着油酸钠浓度的增加,白钨矿浮选回收率先增加后减少,当油酸钠浓度为40 mg·L-1时达到最大值81.72%。图12为不同p H对白钨矿浮选回收率的影响结果。由图12可知,选定油酸钠最佳浓度为40 mg·L-1,随着p H的升高,白钨矿浮选回收率先增加,当p H=10回收率达到最大值95.49%,继续升高p H,白钨矿浮选回收率开始下降。结果表明,在p H=10~11时,白钨矿浮选回收率均在90%以上,p H=10为白钨矿最佳浮选p H值。白钨矿浮选回收率大小顺序为p H=10>p H=11>p H=9>p H=8>p H=12。这与2.4节对应顺序一致,说明白钨矿表面Ca2+会与油酸根离子发生化学吸附,生成油酸钙导致其疏水性增强,从而达到浮选白钨矿的目的。
图1 1 不同油酸钠浓度对白钨矿浮选回收率的影响
Fig.11 Effect of sodium oleate concentration on recovery rate of scheelite flotation
图1 2 不同p H对白钨矿浮选回收率的影响
Fig.12 Effect of p H on recovery rate of scheelite flotation
3结论
1.白钨矿在Na OH溶液中溶解为吸热反应,反应级数n=3。p H=10时溶解反应热Q最大为-24.38m J,p H=12时溶解反应热Q最小为-18.99 m J,溶解反应热大小顺序为p H=10>p H=11>p H=9>p H=8>p H=12,溶解反应热与Ca2+浓度成正相关,在p H=12时溶解反应速率最快。
2.白钨矿在Na OH溶液中吸附油酸钠为放热反应,反应级数n=1,相对白钨矿溶解发生了热量转变,为自发反应。白钨矿吸附油酸钠的反应热大小顺序与白钨矿溶解反应热顺序一致。在p H=10时吸附反应热Q最大为18.77 m J,p H=12时吸附反应热Q最小为16.60 m J,在p H=12时吸附反应速率最快。
3.不同p H条件下油酸根离子的吸附量与其反应热大小成正比,红外光谱测试结果表明油酸根吸附在白钨矿表面与Ca2+生成油酸钙,导致其疏水性增强,且吸收峰强度与吸附量顺序相同,这与浮选结果一致。
参考文献