Article ID: 1003-6326(2005)05-1178-07
A new extraction process of carbonaceous
refractory gold concentrate
MENG Yu-qun(孟宇群)
(Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China)
Abstract:
A new hydrometallurgical process for a carbonaceous refractory gold concentrate at ambient temperature and pressure was presented, including grinding-leaching, intensified alkaline leaching(IAL), thiosulfate leaching and cementation by zinc powder. The experimental results show that the grinding-leaching and intensified alkaline leaching process result in the selective oxidation of arsenopyrite and pyrite. The oxidation ratio of As is 96.6%, and 46.7% for S. The total consumption of NaOH in alkaline leaching is only 28% of that theoretically calculated under the conditions of full oxidization for the same amount of arsenopyrite and pyrite transforming into arsenates and sulfates, and 83.6% of gold is synchro-dissoluted by thiosulfate self-generated during pretreatment. Since the carbonaceous matter in concentrate possesses a strong capability of preg robbing, the cyanidation process is not suitable for the extraction of gold after pretreatment. However, the gold leaching rate by thiosulfate leaching for 24h is increased to 91.7% from 0-3.2% by ultra-fine grinding without the pretreatment. The recovery of gold by zinc cementation gets to 99.6%. Due to the thiosulfate self-generated during alkaline leaching, the reagent addition in thiosulfate leaching afterwards is lower than the normal one.
Key words:
carbonaceous refractory gold concentrate; alkaline leaching; self-leaching of gold; preg robbing; thiosulfate leaching CLC number: TF111.3; TF803.21; TF831;
Document code: A
1 INTRODUCTION
The treatment of refractory gold ores(or concentrates)[1 , 2] attracts more concerns of metallurgists. If there is not only arsenopyrite(FeAsS) bearing gold but also carbonaceous matter contained in the refractory ores, it could be more difficult for the extraction of gold[3-6]. Under many conditions, the carbonaceous matter in refractory ores could possess a strong capability of preg robbing[7-9] so that the cyanide leaching is not suitable for the extraction of gold after pretreatment. Moreover, the cyanide is very toxic. Therefore it is important to develop the non-cyanide method for gold extraction. Thiosulfate is an alternate to cyanide[10-12]. However, due to the high reagent cost as well as requiring heating the pulp[12 , 13] in conventional thiosulfate leaching, its application has been defined to some extent.
In this paper, a hydrometallurgical method is described for the processing of carbonaceous refractory gold-bearing arsenopyrite concentrate under ambient temperature and pressure, including alkaline leaching pretreatment[14-16] and thiosulfate leaching. During alkaline leaching, the oxidation of arsenopyrite and pyrite(FeS2), which always took place under high temperature and pressure, was induced to occur under ambient temperature and pressure by ways of the mechanical activation of an ultra-fine pulverizing-leaching tower mill and the intensification of enhanced agitation tanks. The arsenopyrite was oxidized faster than the pyrite, owing to the lower oxidation potential of FeAsS. Due to the constituent change, lattice distortion and dislocation, and other mineralogical reactions caused by the gold born in minerals, the difference of oxidation distribution happened on the pyrite surface and the pyrite was selectively oxidized as well. Under the condition of low consumption of NaOH, a high oxidation of As (FeAsS was almost completely oxidized), a low oxidation of S(FeS2 was partially oxidized) and a high exposition or liberation of gold born in arsenopyrite and pyrite were got. In the meantime, a portion of gold was synchro-dissoluted by thiosulfate which self-generated during alkaline leaching[14-16].
2 EXPERIMENTAL
2.1 Experimental flowsheet and apparatus
The experimental flowsheet is shown in Fig.1, in which the main apparatus used includes feeder, stirring-type pulverizing-leaching tower mill of d159mm×840mm, enhanced agitation tank of d190mm×285mm, laser particle size analyzer(Mastersizer Ver.2.15), temperature gauge, pH meter, voltameter, air compressor, flow meter, leaching tank, filter, baking oven and XRD.
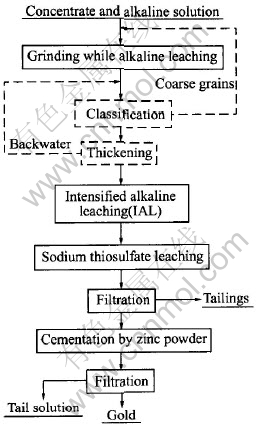
Fig.1 Flowsheet of gold extraction
2.2 Experimental materials
The raw material used in this work was a refractory gold concentrate, produced from Guangxi Province of China, whose chemical composition is shown in Table 1. The carbonaceous matter in the concentrate included the graphite and some organic compounds. The specific mass was 3.17. The gold was not visible, mainly born in FeAsS(78.3%), FeS2(14.2%) and gangue. Under the condition of ultra-fine grinding size (99.9% 〈30.5μm), the dissolution of gold by cyanide was about 0, or only 3.2% by sodium thiosulfate. This is one of the typical refractory gold concentrates.
Table 1 Chemical composition of concentrate (mass fraction, %)

The reagents used were NaOH(IR), NaCN(AR), CuSO4·5H2O(AR), NH3·H2O(AR), Na2SO3(AR), HCl(AR), Na2S2O3·5H2O(AR), CaO(IR), Zn(AR), Fe2(SO4)3(AR) and activated carbon(AR). The water came from the city water pipe.
3 RESULTS AND DISCUSSION
3.1 Grinding and alkaline leaching
The pulverizing-leaching tower mill mainly utilizes the abrasive action to carry out grinding, which possesses not only a strong capability of fine grinding with high efficiency but also a function of mechanical activation. It enables some chemical reactions to be intensified and sped up[14-16] with reduction of the size, and it can be used as a pre-reactor during pulverizing in the fields of activation, cyanidation, alkaline leaching pretreatment, and floatation[14-17].
During alkaline leaching, the active and intensive functions are as follows[14-16]: 1) The coarse arsenopyrite particles are well ground to increase their surface area as well as the active points of reaction; 2) The freshly liberated arsenopyrite particles are subjected to reaction with O2 and NaOH; 3) The enhanced mass transfer conditions increase the contact probabilities among arsenopyrite particles, NaOH and O2; 4) The surface coating and tarnishing are removed from the arsenopyrite particles under the high shear conditions so that the passivation is impossible; 5) The diffusion film is smashed or thinned to reinforce the diffusion;6) The heat generated from grinding and oxidation increases the oxidation rate.
Thus, the combination of grinding-leaching in the tower mill with intensified alkaline leaching in the enhanced agitation tanks can substantially increase the reaction kinetics of alkaline leaching so that the oxidation probabilities of FeAsS, which always takes place under high temperature and pressure, can be induced to occur under ambient ones. The reactions are as follows:
3FeAsS+9NaOH+4O2=
Na3AsS3+2Na3AsO4+3Fe(OH)3(1)
4FeAsS+4FeS2+12NaOH+3O2+6H2O=
4Na3AsS3+8Fe(OH)3(2)
2FeAsS+4NaOH+7O2=
2FeAsO4+2Na2SO4+2H2O(3)
2FeAsS+10NaOH+7O2=
2Fe(OH)3+2Na3AsO4+
2Na2SO4+2H2O(4)
2FeS2+4NaOH+3O2=
2Na2S2O3+2Fe(OH)2(5)
Under certain conditions, the anions S2O2-3 and AsS3-3 can be further oxidized to SO2-4 and AsO3-4, Fe(OH)2 is oxidized to Fe(OH)3, and Fe(OH)3 is decomposed to Fe2O3. The occurring extent of reaction(5) will depend on the reactivity of FeS2.
Under certain conditions, the activated degree of a material corresponds to the grinding size. In this work the grinding size (listed in Table 2) is suitable for the intensified alkaline leaching(IAL) afterwards, which can be economically obtained by the pulverizing-leaching tower mill[16]. When the grinding-leaching is completed, i.e. at the beginning of IAL, the oxidation of As and S are 13.3% and 4.8% in mass fraction, respectively. The contribution to arsenopyrite oxidation by grinding-leaching in the tower mill is large.
3.2 Intensified alkaline leaching(IAL)
3.2.1 Selective oxidation of arsenopyrite and pyrite
After grinding-leaching and with IAL(listed in Table 3) going on in the enhanced agitation tanks, the arsenopyrite and pyrite are selectively oxidized, and the arsenopyrite is oxidized faster than the pyrite. As shown in Fig.2, after IAL for 48h, the oxidation ratios of As and S are 96.6% and 46.7% in mass fraction, respectively.
During IAL, the color of the solid phase changes fast. After IAL for 3h, the solid appears yellow obviously. In the end of IAL, the solid becomes brownish yellow contrasted to the blackish gray of raw material. And the specific mass decreases to 2.61 from 3.17 before pretreatment. Due to the precipitation of Fe3+ and Ca2+ with AsO3-4 in the solution, most of the oxidized As is turned into the solid phase again. In the end of IAL, the concentration of As in solution is 3.8g/L.
Due to the selective oxidation of arsenopyrite and pyrite in IAL, the arsenopyrite is almost completely oxidized, but the pyrite is partially oxidized and the non-oxidized pyrite still keeps its primitive phase. This is further verified by the analysis result of XRD, as shown in Fig.3. After IAL, there is no FeAsS but FeS2 in the solid residue. Thus, most skeletal structures of particles could be reserved[14-16]. As shown in Fig.4, the particle distributions between 10μm and 30μm before and after IAL are well matched. This is profitable for the subsequent extraction of gold.
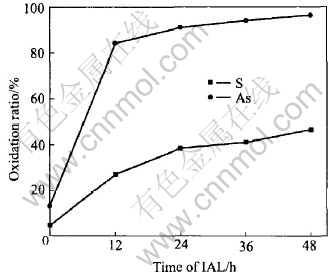
Fig.2 Selective oxidation kinetics of arsenopyrite and pyrite
3.2.2 φh change of pulp
Fig.5 shows the φh change of the pulp. After IAL for 12h, φh rises quickly from initial -378mV to -163mV. Then it increases gradually. After IAL for 48h, it is -97mV. In fact, the changing tendency of φh of the pulp is the objective reflection of the oxidation progress of arsenopyrite and pyrite. The more the arsenopyrite and pyrite are oxidized, the weaker the capacity for the pulp to be oxidized, and the higher the φh of the pulp. At the end of IAL, the refractory concentrate becomes easily extracted for the gold.
3.2.3 Consumption kinetics of NaOH and variation of pulp temperature
As much heat comes out from the oxidation of arsenopyrite and pyrite in IAL, the temperature of pulp rises quickly, as shown in Fig.6. After IAL for 1.25h, the temperature is increased from initial 33℃ to the peak value of 58℃. The rising speed of pulp temperature gets to 20℃/h, and the consumption rate of NaOH for 1t ore reaches the maximum value of 32kg/h. During 1.25-2.5h of IAL, the temperature drops to 45℃ quickly, and the consumption rate of NaOH falls to 8kg/h. After IAL for 3h, both the pulp temperature and the consumption rate of NaOH(about 1.8kg/h) vary slowly. At the end of IAL, the pulp temperature is 42℃.
Table 2 Conditions for grinding-leaching

Table 3 Conditions for intensified alkaline leaching(IAL)

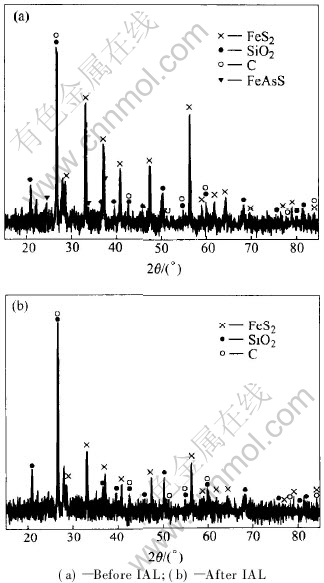
Fig.3 XRD patterns of solids
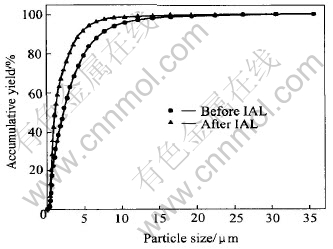
Fig.4 Change of particle distribution after IAL
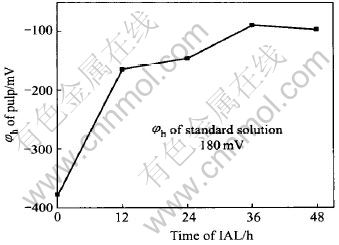
Fig.5 φh change of pulp under pH=11 at 21℃
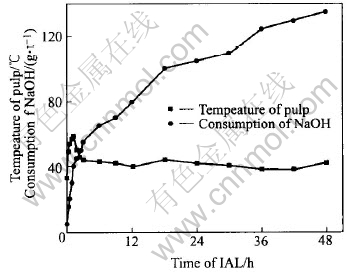
Fig.6 Consumption kinetics of NaOH and variation of pulp temperature
Due to the selective oxidation of arsenopyrite and pyrite as well as the generation of thiosalts shown in reactions(1),(2) and(5) during alkaline leaching, the amount of sulfur which needs to be fully oxidized is decreased and a smaller amount of NaOH is required. After IAL for 48h, the total consumption of NaOH for 1t ore is 135kg that includes 5kg NaOH consumed during the grinding-leaching process. This is only 28% of those theoretically calculated under the conditions of full oxidization for the same amount of arsenopyrite and pyrite transforming into arsenates and sulfates.
3.2.4 Concentration change of S2O2-3 in solution and self-leaching of gold
The thiosulfate is a good and non-toxic reagent for the extraction of gold[10-13]. During alkaline leaching, some thiosalts, including thiosulfate, can be generated in the oxidation of arsenopyrite and pyrite(shown in reactions(1),(2) and(5)). With the alkaline leaching going on, the gold born in arsenopyrite and pyrite is progressively exposed or liberated, and simultaneously some of them can be synchro-dissoluted by the self-generated thiosulfate. This phenomenon is called synchro-self-leaching of gold during alkaline leaching pretreatment[14-16].
Fig.7 gives the concentration change of S2O2-3 in solution and the result of synchro-self-leaching of gold during alkaline leaching. When the grinding-leaching is completed, i.e. at the beginning of IAL, the concentration of S2O2-3 in solution is 0.98g/L, and the self-leaching rate of gold is 10.6%. After IAL for 12h, the concentration of S2O2-3 in solution rises to 36.2g/L quickly, and the self-leaching rate of gold is 65.7%. Then, the self-leaching rate of gold slowly increases to 83.6% in the end. However, for the contentration of S2O2-3 in solution, after it reaches the maximum value of 57.9g/L for 36h IAL, it drops a little to 50.4g/L in the end. This is because that the amount of sulfur oxidized during 36-48h IAL was much less(only 5.6%) and the oxidation speed of S2O2-3 to SO2-4 is higher than its generation rate. Anyhow, this little decrease for the concentration of S2O2-3 in solution does not affect the self-leaching of gold under the conditions of IAL.

Fig.7 Concentration change of S2O2-3 in solution and corresponding self-leaching rate of gold
3.3 Gold extraction
3.3.1 Preg robbing of carbonaceous matter in cyanidation
Some activated carbon ingredients in the carbonaceous matter can adsorb the gold from the cyanidation solution, resulting in a low rate of recovery of leaching. This behavior is called preg robbing[7]. After pretreatment, the action of preg robbing is tested in cyanidation, as shown in Fig.8. For 3h cyanide leaching, the leaching recovery of gold gets to the maximum value of 89.0%. Afterwards, it drops gradually. For 24h cyanide leaching, it further drops to 79.6%, which is lower than the self-leaching rate of 83.6% during alkaline leaching pretreatment. These results show that the carbonaceous matter in the concentrate possesses a strong capability of preg robbing.
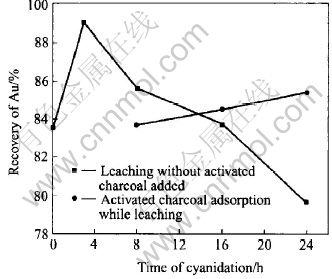
Fig.8 Preg robbing of carbonaceous matter in cyanidation
If 30g/L activated carbon is added in the pulp at the beginning of cyanidation in order to generate a competitive adsorption together with the carbonaceous matter, the effect of preg robbing could be cut down to some extent. However, the recovery of gold by activated carbon for 24h absorption while cyanide leaching is 84.7%, only 1.1% higher than the self-leaching rate during pretreatment and yet is lower than the maximum cyanide leaching rate of 89.0% after pretreatment. It is shown that the competitive adsorption of activated carbon could not completely eliminate the preg robbing effect of carbonaceous matter. Moreover, the consumption of NaCN is very high, about 18kg/t.
Based on the results above, it is shown that the cyanidation process is not suitable for the extraction of gold from the oxidized pulp.
3.3.2 Sodium thiosulfate leaching
After pretreatment, the recovery of gold by cyanide and activated carbon is low because of the preg robbing effect of carbonaceous matter in the gold-bearing materials. Besides, the cyanide is very toxic, which can cause serious poison problem to the environment. Therefore it is important to develop the non-cyanide method for gold extraction. The thiosulfate is a non-toxic reagent that possesses a strong capability of gold extracting from ores[10-13]. Different from the anions Au(CN)-2, the anions Au(S2O3)-32 cannot be adsorbed by carbonaceous matter[12]. Since there is an amount of self-generated thiosulfate and a portion of synchro-dissoluted gold during alkaline leaching, it might be more reasonable for thiosulfate leaching process just following the pretreatment. On one hand this can eliminate the preg robbing effect of carbonaceous matter in the gold-bearing materials; on the other hand it can result in not only a non-cyanide extraction of gold but also a less reagent consumption. In fact, due to the main defect of high reagent cost in conventional thiosulfate leaching, its application has been defined to some extent[12 , 13].
Under the conditions listed in Table 4, Fig.9 shows the dissolution results by an additional Na2S2O3 in the oxidized pulp for 24h leaching at the ambient temperature of 21-24℃. When the addition of Na2S2O3 in solution increases to 0.11mol/L, the dissolution of gold is the highest, reaching 91.7%. When it further increases to 0.22mol/L, the dissolution of gold drops a little to 88.6%. More addition of Na2S2O3 is disadvantageous to the gold extraction. This is because that it can cause the stabilizing equilibrium of reaction(6) generated by the additional Na2SO3 to move towards the left:
6H++4SO2-3+2S-2=3S2O2-3+3H2O(6)
However, the anions S-2 could give rise to the sulfide film precipitated on the surface of gold, impeding the extraction process. Moreover, it is noticeable that the dissolution of gold under the condition of no Na2S2O3 addition in the pulp is only 86.6%. These results show that a high dissolution of gold can be obtained at ambient temperature without heating the pulp in contrast with leaching at 40-60 ℃ for some other conventional thiosulfate methods[10-13], and the consumptions of Na2S2O3, CuSO4, NH3·H2O and Na2SO3 listed in Table 4 are several times lower than those in other thiosulfate leaching processes[10-13].
Table 4 Conditions for conventional mixing of sodium thiosulfate leaching
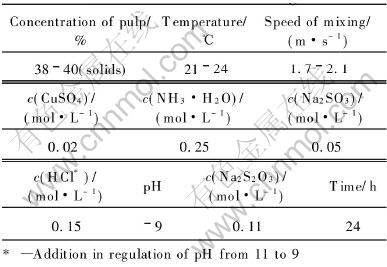
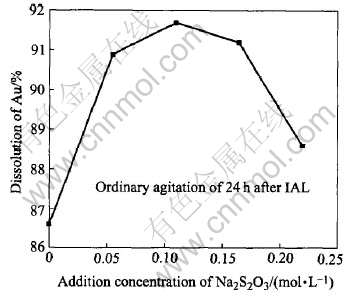
Fig.9 Sodium thiosulfate leaching of gold
3.3.3 Cementation by zinc powder
After thiosulfate leaching, if 30g/L activated carbon is added in the filtrate, the adsorption of gold for 24h is zero. This further confirms the point of view that the anions Au(S2O3)3-2 can not be adsorbed by carbonaceous matter[14]. However, when 0.18kg/m3 zinc powder is added in the filtrate, the recovery of gold by zinc cementation for 0.5h gets to 99.6%.
3.4 Environmental control and techno-economic assessment
After zinc cementation and filtration, 6.5kg/m3 Fe2(SO4)3 is added step-by-step to the solution in a conventional agitation tank. The arsenide in the solution is precipitated to form a stable phase of ferric arsenate. The concentration of As in the solution is decreased below 0.5mg/L, which meets the requirement of the environmental pollution control system.
A preliminary techno-economic assessment shows that processing of the objective concentrate is about RMB 430 yuan per ton.
4 CONCLUSIONS
1) After grinding-leaching in a pulverizing-leaching tower mill and intensified alkaline leaching for 48h in the enhanced agitation tanks at ambient temperature(21-24℃) and pressure(105Pa), the dissolution of gold by thiosulfate of the objective carbonaceous refractory concentrate is enhanced to 91.7% from 0-3.2% by ultra-fine grinding without the pretreatment, and the recovery of gold by zinc powder cementation in the solution gets to 99.6%.
2) Due to the selective oxidation of arsenopyrite and pyrite as well as the generation of thiosalts during alkaline leaching, the total consumption of NaOH in the present processing is only 28% of those theoretically calculated under the conditions of full oxidization for the same amount of arsenopyrite and pyrite transforming into arsenates and sulfates. And the thiosulfate self-generated during alkaline leaching results in 83.6% of gold synchro-dissoluted during the pretreatment.
3) The carbonaceous matter in the gold-bearing materials possesses a strong capability of preg robbing. The cyanidation process is not suitable for the extraction of gold after the pretreatment. However, small addition of thiosulfate afterwards can make a high recovery of gold at ambient temperature without pulp heating.
REFERENCES
[1]Vaughan J P. The process mineralogy of gold: the classification of ore types [J]. JOM, 2004, 56(7): 46-48.
[2]Zhou J Y, Cabri L J. Gold process mineralogy: objectives, techniques, and applications [J]. JOM, 2004, 56(7): 49-52.
[3]Afenya P M. Treatment of carbonaceous refractory gold ores [J]. Minerals Engineering, 1991, 4(7-11): 1043-1055.
[4]Adams M D, Burger A M. Characterization and blinding of carbonaceous preg-robbers in gold ores [J]. Minerals Engineering, 1998, 11(10): 919-927.
[5]Liu Y, Liu Q. Flotation separation of carbonate from sulfide minerals, I: flotation of single minerals and mineral mixtures [J]. Minerals Engineering, 2004, 17(7-8): 855-863.
[6]Stenebraten J F, Johnson W P, Brosnahan D R. Characterization of goldstrike ore carbonaceous material—Part 1: chemical characteristics [J]. Mineral & Metallurgical Processing, 1999, 16(3): 37-43.
[7]Hausen D M, Bucknam C H. Study of preg robbing in the cyanidation of carbonaceous gold ores from carlin [A]. Proceedings of the Second International Congress on Applied Mineralogy [C]. Nevada, USA, 1985. 833-856.
[8]WU Min-jie, BAI Chun-gen. Carbonaceous matter in carbonaceous gold ores: its material composition and interaction with gold [J]. Gold, 1994, 15(6): 29-35.(in Chinese)
[9]Lagerge S, Zajac J, Partyka S, et al. Comparative study on the adsorption of cyanide gold complexes onto different carbonaceous samples: measurement of the reversibility of the process and assessment of the active surface inferred by flow microcalorimetry [J]. Langmuir, 1999, 15(14): 4803-4811.
[10]HUANG Wan-fu, WANG Dian-zuo, HU Yong-ping. Theory and practice of leaching gold by thiosulfate [J]. Gold, 1998, 19(9): 34-36.(in Chinese)
[11]Muir D M, Aylmore M G. Thiosulphate as an alternative to cyanide for gold processing—issues and impediments [J]. Transactions of the Institution of Ming and Metallurgy Section C—Mineral Processing and Extractive Metallurgy, 2004, 113(1): 2-12.
[12]Aylmore M G, Muir D G. Thiosulfate leaching of gold—a review [J]. Minerals Engineering, 2001, 14(2): 135-174.
[13]Xia C, Yen W T, Deschenes G. Improvement of thiosulfate stability in gold leaching [J]. 2003, 20(2): 68-72.
[14]MENG Yu-qun, WU Min-jie, SU Shao-ling, et al. Intensified alkaline leaching pretreatment of refractory gold concentrates at common temperature and pressure [J]. Trans Nonferrous Met Soc China, 2003, 13(2): 426-430.
[15]MENG Yu-qun, JIN Tao, WU Min-jie, et al. A new extraction process of refractory gold concentrates [J]. Journal of University of Science and Technology Beijing, 2003, 10(5): 9-14.
[16]MENG Yu-qun. Research on the Principle of Intensified Alkaline Leaching Process and the Key Industrial Application Techniques for Refractory Gold—Bearing Materials [D]. Graduate School of the Chinese Academy of Sciences, Beijing, 2003.(in Chinese)
[17]MENG Yu-qun, WU Min-jie, SU Shao-ling, et al. Experimental research on a new process of flotation while grinding [J]. Mining and Metallurgical Engineering, 2003, 23(1): 28-30.(in Chinese)
Foundation item: Project(2001101015) supported by the Natural Science Foundation of Liaoning Province of China; Project(AM05-0866) supported by the Free Study Item of Institute of Metal Research, Chinese Academy of Sciences
Received date: 2004-10-28; Accepted date:2005-05-15
Correspondence: MENG Yu-qun, Senior Engineer, PhD; Tel: +86-24-23971931, Fax: +86-24-23971931; E-mail: yqmeng@imr.ac.cn


