网络首发时间: 2016-06-22 08:23
掺氮纳米金刚石薄膜的微观结构对微波场发射性能影响
西南科技大学四川省非金属复合与功能材料重点实验室—省部共建国家重点实验室培育地
摘 要:
采用微波等离子体化学气相沉积(MPCVD)方法,通过改变CH_4浓度,在单晶Si(100)基底上制备掺氮纳米金刚石(NCD)薄膜,并以所制备的掺氮NCD薄膜为阴极材料,通过场发射扫描电子显微镜(FESEM)、原子力扫描探针显微镜(AFM)、Raman光谱和S波段射频电子枪等测试方法系统地研究了掺氮NCD薄膜的微观结构对微波场发射性能的影响。结果表明:在CH_4浓度(体积比)为4%下,制备的掺氮NCD薄膜的颗粒呈多面体,而且颗粒尺寸和表面粗糙度较大,薄膜中金刚石相含量较高,这些微观结构使得微波场发射性能较高,在电场强度(E_0)为67.7 V·μm~(-1)时,发射电流密度(J0)高达144.8 m A·cm~(-2)。当升高CH_4浓度,所制备的掺氮NCD薄膜的颗粒尺寸减小而且连成条状结构,表面粗糙度也逐渐降低,薄膜中金刚石相减少、非金刚石相增加,这些微观结构的改变使得微波场发射性能逐渐降低。如当CH_4浓度增加至6%时,在电场强度E_0=67.7 V·μm~(-1)时,场发射电流密度降至37.9 m A·cm~(-2)。结果表明:低CH_4浓度下,掺氮NCD薄膜所具有的微观结构有利于微波场发射。
关键词:
微波等离子体化学气相沉积;CH4浓度;掺氮纳米金刚石薄膜;微观结构;微波场发射;
中图分类号: TB383.2
作者简介:许立(1987-),男,河南信阳人,硕士研究生,研究方向:CVD金刚石薄膜制备与研究;E-mail:xyzphysics@163.com;;熊鹰,教授;电话:13508108924;E-mail:xiongying@swust.edu.cn;
收稿日期:2015-01-05
基金:国家自然科学基金委员会与中国工程物理研究院联合基金项目(U1330127);国家自然科学基金青年基金项目(11205127)资助;
Microwave Field Emission Performances of Nitrogen-Doped NCD Thin Films Affected by Microstructure
Xu Li Xiong Ying Wang Bing Han Wenhong Liu Xinglong Fang Liping
State Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials,Southwest University of Science and Technology
Abstract:
Nitrogen-doped nanocrystalline diamond( NCD) thin films were deposited on single crystal Si( 100) substrates by microwave plasma chemical vapor deposition( MPCVD) with different CH_4 concentrations. With nitrogen-doped NCD films as cathode materials,the effect of the microstructure of NCD thin films on microwave field emission was systematically studied by field emission scanning electron microscopy( FESEM),atomic force scanning probe microscope( AFM),Raman spectrum and the S-band radio frequency gun. The results showed that under 4% CH_4concentration( volume ratio),the nitrogen-doped NCD particle shapes were polyhedron,the grain size and the surface roughness were larger,and the diamond phase content in the diamond film was higher than those of other samples. The above microstructure had excellent field emission properties,for example,the emission current density J0= 144. 8 m A·cm~(-2)at 67. 7 V·μm~(-1)electric field. With the increase of CH_4 concentration,the grain size and surface roughness of NCD thin films would gradually decrease and the microstructure changed from polyhedron to strip structure. The film's diamond phase decreased and its non-diamond phase increased. The microwave field emission properties decayed with the microstructure change. Under 6% CH_4 concentration,the emission current density( J0) decreased to 37. 9 m A·cm~(-2)at 67. 7 V·μm~(-1)electric field. The results showed that the microstructure of nitrogen-doped NCD film was advantageous to the field emission properties under low CH_4 concentration.
Keyword:
microwave plasma chemical vapor deposition(MPCVD); CH4 concentration; nitrogen-doped NCD thin films; microstructure; microwave field emission;
Received: 2015-01-05
纳米金刚石(NCD)薄膜主要是由纳米级金刚石颗粒和无定性碳组成的,且颗粒尺寸在100 nm以下。相比于纯金刚石相,纳米金刚石薄膜具有更优异的性能而吸引着大量学者的研究
基于场发射的冷阴极微波射频电子枪兼具光阴极和热阴极微波电子枪的优点,可提供低发射度、低能散和较高流强的电子束
目前国内外用该微波射频电子枪在金刚石薄膜方面的实验报道很少
1 实验
1.1 掺氮NCD薄膜的制备
为促进金刚石薄膜在基底的形核,需要在制备掺氮NCD薄膜之前对异质基底进行处理。具体工作如下:将直径为6 mm的圆形单晶硅片放入浓硫酸和浓双氧水的混合溶液(体积比为3∶1)搅拌处理30 min,并取出用蒸馏水清洗样品,再用N2吹干;然后放入超NCD粉悬浮液中超声30 min以形核,最后用蒸馏水清洗,用N2吹干。
掺氮NCD薄膜的沉积采用自行研制的10 k W微波等离子体化学气相沉积(MPCVD)系统。具体工艺参数如下:沉积微波功率为6 k W,腔体工作气压为14.3 k Pa,反应气源为Ar,CH4和CO2,气体总流量为500 ml·min-1,其中CH4的流量为4%~6%(体积分数),CO2的流量为6 ml·min-1(用作载气将三聚氰胺的甲醇饱和溶液载入到腔体中作为液态掺氮源)。利用红外测温仪测得掺氮NCD薄膜的生长温度为860℃,所有掺氮NCD薄膜的沉积时间均为4 h。
在此基础之上,保持微波功率、工作压力以及CO2载气流量等参数不变,通过改变CH4浓度制备3种掺氮NCD薄膜,称为样品1,2,3分别对应CH4浓度为4%,5%和6%,并以场发射扫描电镜(FESEM)和Raman测试方法研究不同CH4浓度对掺氮NCD薄膜微观结构的改变,并通过S波段射频电子枪测试平台研究微波场发射性能随薄膜微观结构的改变。
1.2 测试与表征
通过Zeiss Ultra55型FESEM对制备的掺氮NCD薄膜的形态和微观结构进行表征;使用514.5nm波长的In Via型激光Raman(Reinshaw公司)光谱仪测试样品成分结构。
1.3 S波段微波场发射测试
使用的场发射平台为中国工程物理研究院应用电子学研究所的S波段射频电子枪
S波段微波场发射性能的测试数据为平均电流(I)与电压(U)
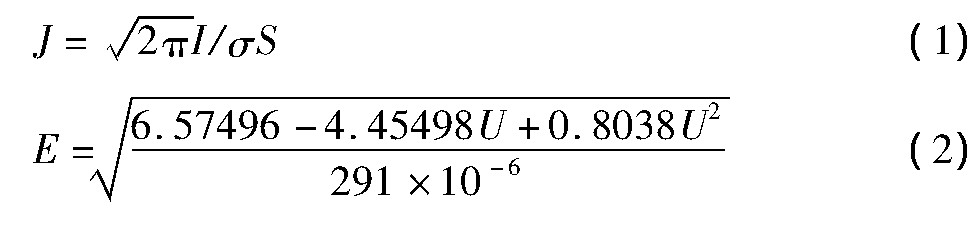
式中σ为均方根宽度,在该测试平台上,对于金刚石的均方根宽度约为12°,S为NCD薄膜阴极的发射面积,其有效发射直径为5 mm。
2 结果与讨论
2.1 NCD薄膜微观结构的表征与分析
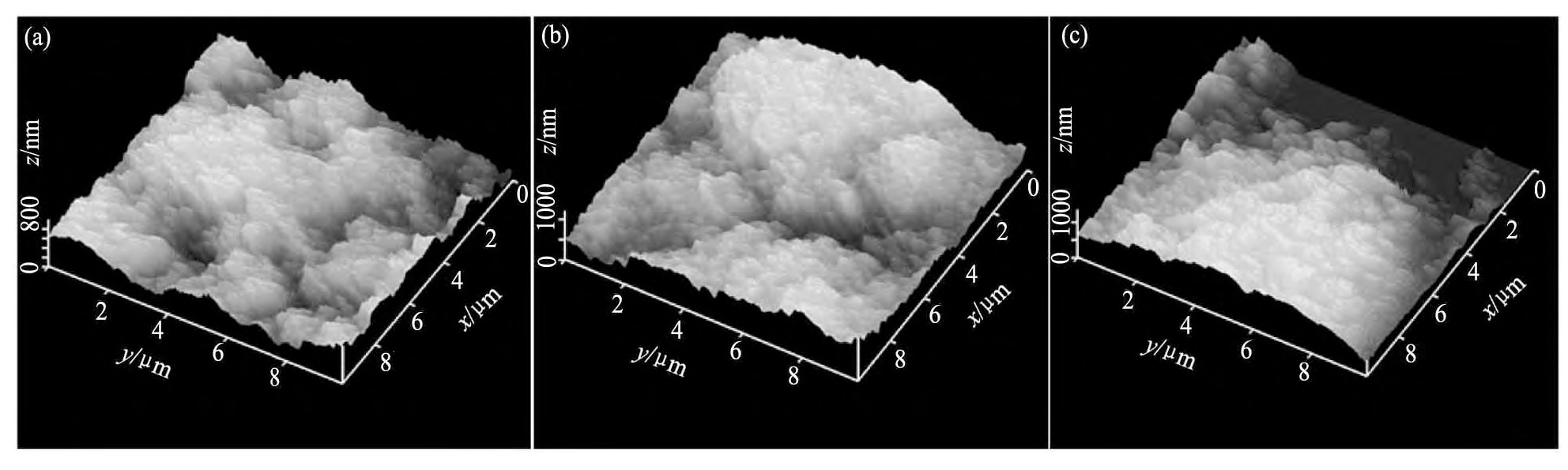
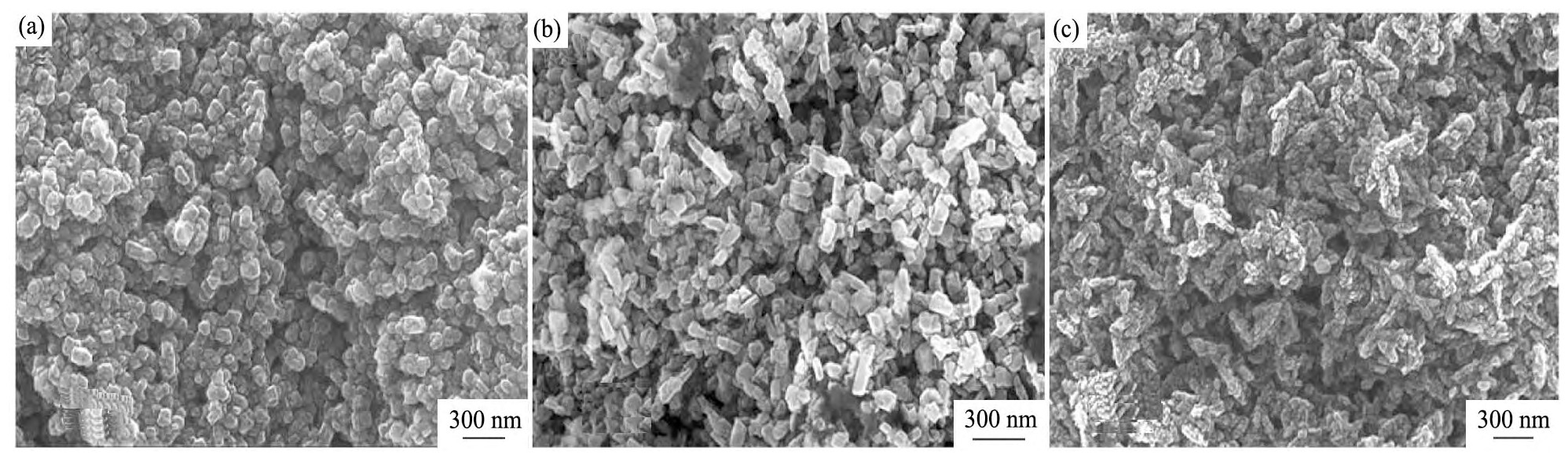
图1为不同CH4浓度制备的掺氮NCD薄膜的原子力扫描探针显微镜(AFM)图。从图1中可以看出随着CH4浓度由4%不断增加到6%时,所制备的掺氮NCD薄膜表面形态也随着发生明显的改变。在测试范围10μm×10μm内,掺氮NCD薄膜样品1,2,3的表面粗糙度分别为175,110,104nm。图2为该不同CH4浓度下的FESEM图,如图2(a),所制备的薄膜由尺寸为100 nm左右的NCD颗粒聚集而成,这些颗粒呈近视规则的多面体形状。当CH4浓度增加至5%时,如图2(b)所示,制备的NCD薄膜仍由多面体状的NCD颗粒聚集而成,但NCD颗粒呈细长状,且尺寸略有下降。当CH4浓度继续增加至6%时,如图2(c)所示,NCD薄膜中NCD颗粒的尺寸明显变小,这些细小的纳米颗粒聚集成长约200 nm,宽约20~50 nm的条状结构,这与Chen等
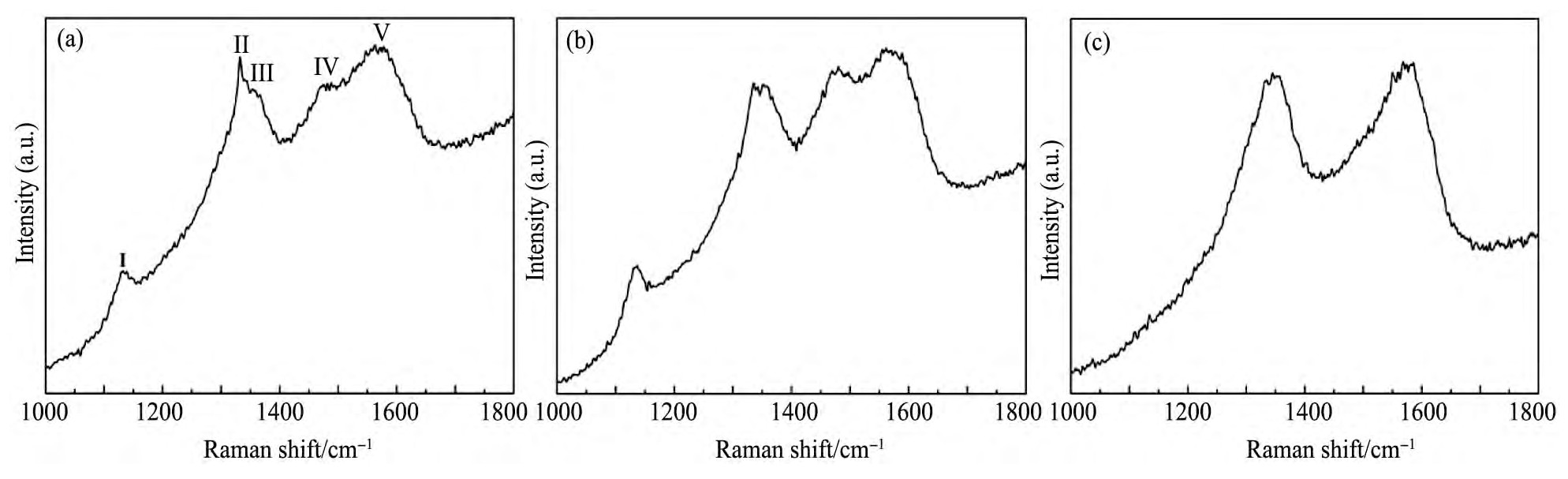
图3为不同CH4浓度下所制备的掺氮NCD薄膜的Raman光谱图。激光Raman光谱对碳键十分敏感,在区分金刚石、石墨、非晶碳和碳氢物质中的碳结构远远优于其他表征手段
图1 不同CH4浓度下制备的掺氮NCD薄膜AFM照片Fig.1 AFM images of nitrogen-doped NCD thin films deposited at different CH4concentrations
(a)4%CH4;(b)5%CH4;(c)6%CH4
图2 不同CH4浓度下制备的掺氮NCD薄膜的FESEM照片Fig.2 FESEM images of nitrogen-doped NCD thin films deposited at different CH4concentrations
(a)4%CH4;(b)5%CH4;(c)6%CH4
图3 不同CH4浓度下制备的掺氮NCD薄膜的Raman光谱Fig.3 Raman spectra of nitrogen-doped NCD thin films deposited at different CH4concentrations
(a)4%CH4;(b)5%CH4;(c)6%CH4
从以上的FESEM,AFM和Raman结果可以得出,随着CH4浓度增加,金刚石薄膜的微观结构发生如下变化:金刚石晶粒的尺寸逐渐降低,石墨相的含量逐渐增加;同时NCD薄膜中金刚石的形貌逐渐由多面体状纳米颗粒逐渐向长条状结构转变。一般来说,在MPCVD沉积金刚石薄膜的过程中,等离子体中的含碳基团如CH-,C2以及原子氢等之间的含量将直接影响金刚石相或石墨相的沉积和刻蚀速率,从而影响最终所制备的薄膜的微观结构。在较低的CH4浓度下,由于烃类(CH-)基团的浓度较高,而有利于提高金刚石二次形核率的C2基团浓度较低,因此在该条件下所制备的NCD薄膜的金刚石晶粒尺寸较大,且石墨含量较少。随着CH4浓度的增加,等离子体中的C2基团浓度增加,因此等离子体中的C2/H浓度比逐渐增加,而CH/C2浓度比逐渐减少,这有利于薄膜生长中金刚石的二次形核,从而导致金刚石晶粒的尺寸逐渐降低,石墨相的含量逐渐增加
2.2 NCD薄膜的S波段微波场发射性能及分析
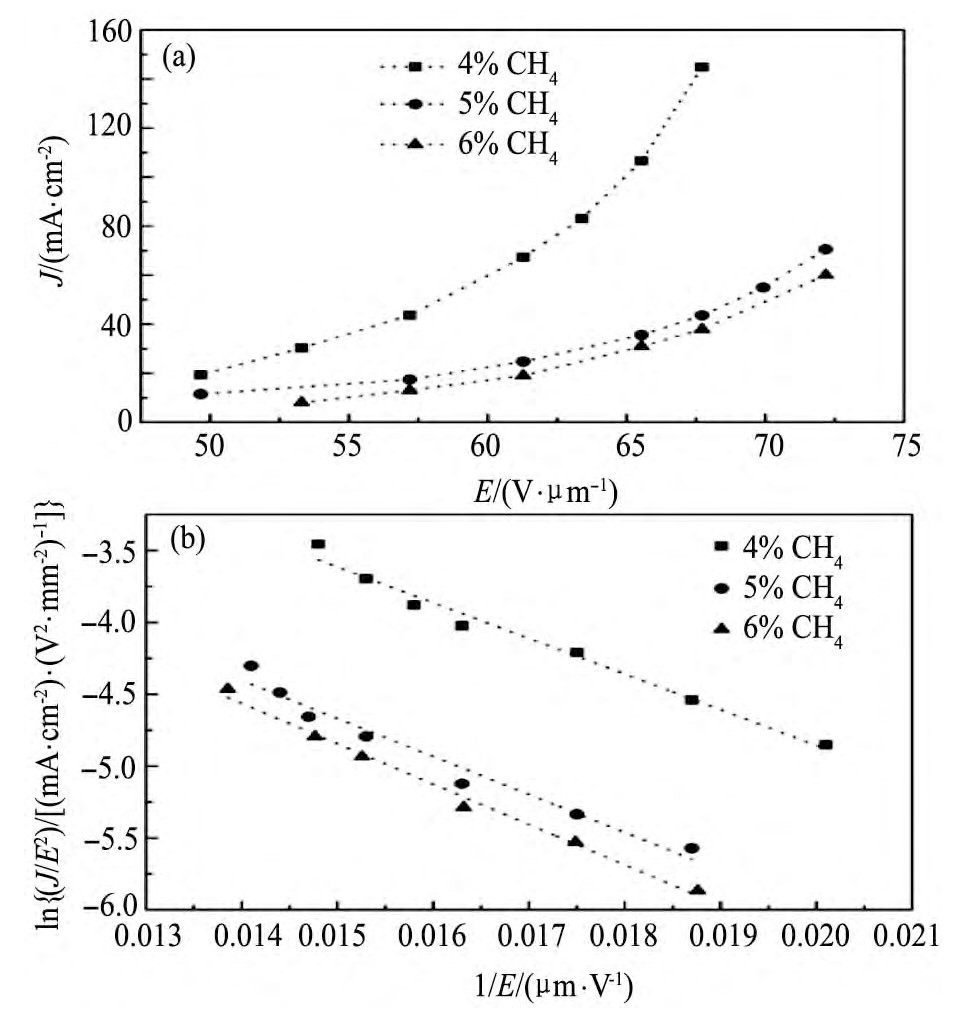
在中国工程物理研究院的S波段射频电子枪测试平台上,以不同微观结构的掺氮NCD薄膜为阴极,测试NCD薄膜的场发射性能。图4(a)给出了掺氮NCD薄膜阴极的发射电流密度值(J)和微波场电场强度(E)之间的关系曲线。可以看出,CH4浓度较低的掺氮NCD薄膜(4%)的发射电流密度随着微波场电场强度的增加而快速的增加,如当电场强度从49.6 V·μm-1增加到67.7 V·μm-1时,发射电流密度从19.3 m A·cm-2迅速增加至144.8m A·cm-2。而其他两个较高CH4浓度的掺氮NCD薄膜(5%和6%)的发射电流密度的变化趋势基本相似,即随着微波场电场强度的增加而缓慢增加,如当电场强度增加至67.7 V·μm-1时,发射电流密度也分别仅为43.6和37.9 m A·cm-2。以上结果表明:掺氮NCD薄膜的表面形貌对S波段微波场发射性能有着重要的影响,即降低CH4浓度使得薄膜中的金刚石颗粒尺寸增大,表面粗糙的增大,晶界处的石墨相含量减少,这些形貌的改变有利于提高所制备的掺氮NCD薄膜的S波段微波场场发射性能。最近,Baryshev等
图4 不同CH4浓度下掺氮NCD薄膜的场发射电流密度(J)与电场强度(E)曲线以及F-N拟合曲线Fig.4 Emission current density vs.electrical field curves(a)and fitted F-N plots of nitrogen-doped NCD thin films deposited at different CH4concentrations(b)
2.3 微观结构对微波场发射性能的影响
根据Fowler-Nordheim(F-N)法则和发射电流密度-电场强度曲线,也拟合出了3个掺氮NCD薄膜阴极场发射的F-N曲线,如图4(b)所示。在测试的整个电场强度范围(49.6~72.2 V·μm-1)内,所有样品的F-N曲线均为直线,且直线的斜率几乎相等,表明这些样品的发射遵循经典的场致发射电子特征规律。由F-N曲线的斜率和截距,可以进一步计算出以上3个样品的场增强因子(β),随着CH4浓度的增加,样品的场增强因子逐渐从约18.5降低至11.3左右,这与AFM测试结果具有很好的一致性。如表1所示:4%CH4浓度下NCD薄膜的场增强因子和均方面粗糙度(RMS)都要比5%和6%高很多,且5%与6%CH4浓度下的值比较相近。这可以很好地解释以上3种样品场发射性能差异的现象。即由于4%CH4浓度下NCD薄膜样品的表面有许多不平整的凸起尖端,在尖端效应的作用下,金刚石薄膜尖端处会产生很强的局部电场,进而增强了薄膜的场发射性能。
表1 不同CH4浓度掺氮NCD薄膜的微波场发射和表面形貌参数Table 1 Parameters of microwave field emission and sur-face morphology of nitrogen-doped NCD thin films deposited at different CH4concentrations 下载原图
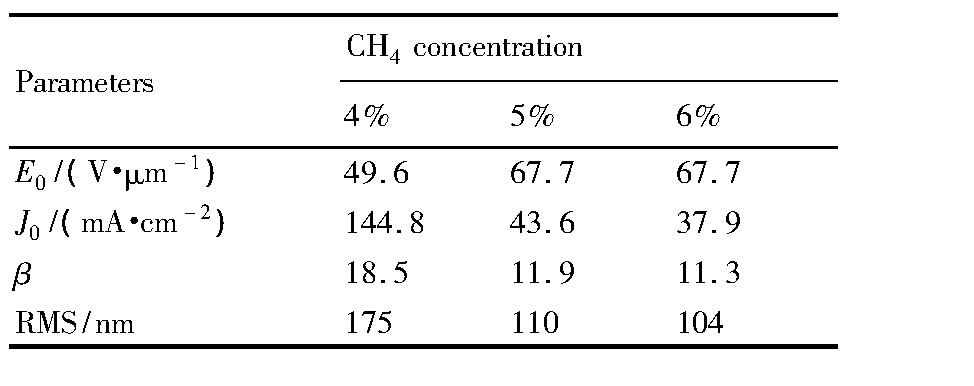
表1 不同CH4浓度掺氮NCD薄膜的微波场发射和表面形貌参数Table 1 Parameters of microwave field emission and sur-face morphology of nitrogen-doped NCD thin films deposited at different CH4concentrations
3 结论
研究了CH4浓度对MPCVD方法生长的掺氮NCD薄膜的表面形貌、表面粗糙度和物相等微观结构的改变,进而对微波场发射的影响。即掺氮NCD薄膜的微观结构随着CH4浓度的变化而发生变化,降低CH4浓度导致薄膜中金刚石相含量增加,晶界处的非金刚石相较少;金刚石薄膜的颗粒形状也逐渐由长条状向多面体变化,而且颗粒尺寸和表面粗糙度也跟着增大;结合微波场发射测试结果表明:低CH4浓度下制备的掺氮NCD薄膜具有的微观结构可以获得更高的场发射电流密度。如在整个测试电场范围内,电流密度最高可达144.8 m A·cm-2(电场E=67.7 V·μm-1)。在高、低加速梯度的加速器应用程序中,鉴于金刚石薄膜的场发射性能方面的优点,很有可能作为一种优良、高效和稳定的场发射阴极。
参考文献
[11] Tuinstra F,Koenig J L,Chem J.Raman spectrum of graphite[J].Phys.,1970,53(3):1126.