文章编号:1004-0609(2010)06-1083-05
Al-Mg-Sc-Zr合金中初生相的析出行为
杜 刚,杨 文,闫德胜,戎利建
(中国科学院 金属研究所,沈阳 110016)
摘 要:
利用扫描电子显微镜(SEM)和电子探针(EPMA)研究液态金属的冷却速率对Al-6Mg-0.2Sc-0.15Zr(质量分数,%)合金中初生相的结构、形貌及成分的影响。结果表明:在较低的冷却速率下(随炉冷却),液态金属中析出的初生相为L12结构的Al3(Sc, Zr)相和D023结构的Al3(Zr, Sc)相。初生Al3(Sc, Zr)相为Zr溶解在Al3Sc相中的固溶体,具有复杂的形貌和较高的体积分数;当冷却速率较大时(钢模具冷却),D023结构的Al3(Zr, Sc)相的析出受到抑制而形成L12结构的Al3(Sc, Zr)相或亚稳态的Al3(Zr, Sc)相;当冷却速率足够大时(铜模具冷却),α(Al)基体在较高的过冷度下快速结晶,初生相的形成完全受到抑制。
关键词:
铝合金;Al-Mg-Sc-Zr合金;初生相;冷却速率;显微组织;
中图分类号:TG146.2 文献标志码:A
Precipitation behaviors of primary phases in Al-Mg-Sc-Zr alloy
DU Gang, YANG Wen, YAN De-sheng, RONG Li-jian
(Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China)
Abstract: The effects of cooling rate on microstructures, morphology and composition of the primary precipitates in an Al-6Mg-0.2Sc-0.15Zr alloy were studied by SEM and EPMA. The results show that, at slower cooling rate (furnace cooling), the primary precipitates consist of L12 structured Al3(Sc, Zr) and D023 structured Al3(Zr, Sc) phases, which have complex shapes and higher volume fraction. At moderate cooling rate (iron mould), the D023 structured Al3(Zr, Sc) is suppressed while cubic and L12 structured Al3(Sc, Zr) or metastable Al3(Zr, Sc) form, which have lower volume fraction but higher number density. At rapid cooling rate (copper mould), the α(Al) quickly solidifies due to the large undercooling while the formation of precipitate is suppressed.
Key words: aluminium alloy; Al-Mg-Sc-Zr alloy; primary phase; cooling rate; microstructure
合金元素Sc具有强烈细化Al合金晶粒的作用。当Al合金中Sc的含量超过共晶成分时,液相中能够形成大量的初生Al3Sc相颗粒并成为α(Al)基体的异质形核中心[1-3]。加入Zr或Ti后不但明显降低形成初生相所需的最低Sc含量还可以获得更好的细化晶粒的效果[4-5]。YIN等[6]研究发现铸态Al-5Mg-0.2Sc- 0.1Zr(质量分数,%) 合金的晶粒尺寸可被细化至42 μm,而Al-5Mg-0.6Sc(质量分数,%) 合金的平均晶粒尺寸为72 μm。
Al3Sc/Al3(Sc,Zr)初生相的形成对于Al合金的组织和力学性能具有重要的意义。首先,初生相细化晶粒的能力要受到其结构[2-3]、形貌和尺寸等因素影响。其次,通过控制初生相的析出行为还可以将更多的Sc和Zr元素固溶在α(Al)基体中,从而在随后的形变热处理过程中析出更多的二次Al3 (Sc, Zr)相颗粒以提高变形合金强度和再结晶温度[7-9]。另外,初生相的生成也会对Al-Mg合金的耐腐蚀性和塑性产生影响[10-11]。
目前,已有研究结果表明凝固过程中液态金属的冷却速率能够对Al-Sc, Al-Zr二元合金中Al3Sc, Al3Zr相的形貌产生影响[2-3, 12]。但是,冷却速率对Al-Mg-Sc-Zr四元合金中初生相析出行为的影响目前尚不清楚。本文作者通过设计不同的冷却条件研究冷却速率对Al-Mg-Sc-Zr合金中初生相的结构,形貌和成分的影响。
1 实验
实验合金成分为Al-6Mg-0.2Sc-0.15Zr(质量分数,%),铝液经过精炼后分别在3种冷却条件下凝固:1)在720 ℃下将200 g铝液浇入氧化铝坩埚中,坩埚置于箱式电阻炉中保温10 min后随炉冷却(合金1),冷却速率约为2 ℃/min;2) 铝液在720 ℃下浇入预热钢模具中获得质量为15 kg的铸锭(合金2);3) 铝液在720 ℃下浇入铜模具中获得尺寸为23 mm×23 mm×40 mm的铸锭(合金3)。
采用扫描电子显微镜(SEM)对铸态组织中的初生相形貌进行观察。初生相中的元素分布在EPMA-1610型电子探针上进行分析,并通过能谱仪(EDX)进行定量分析。为观察各种初生相的表面形貌,对金相观察后的试样进行过腐蚀处理。腐蚀液为2.5 mL HNO3,1.5 mL HCl,1.0 mL HF,100 mL H2O,浸蚀时间约为5 min。在Rigaku D/Max-2500PC型衍射仪上进行结构分析,所用试样均为过腐蚀处理后的试样。
2 结果与讨论
由于合金中Sc和Zr的含量较少,试样经过腐蚀后进行XRD测试以提高初生相的衍射强度,因此,得到的初生相的体积分数高于实际水平。图1所示为炉冷合金铸态组织中初生相的XRD谱和形貌。从衍射结果(图1(a))可以看出,合金1中生成了L12和D023结构的两种初生相。根据成分和形貌的可将初生相分为3类。图1(b)中长条形的白色析出相为D023结构的Al3(Zr,Sc)相。图2所示为合金1中初生相的EPMA结果。从图2中可看出,该相中的含量较高,并含有微量Sc,EDX分析结果表明该相中Sc的含量低于1%(质量分数,%),由此可将此类相看作是微量Sc溶解在Al3 Zr中的固溶体。图1(b)中颜色较暗的析出相为Al3Sc,由于该相中几乎不含有Zr原子因而衬度较暗。其余形状较为复杂的初生相,其中的Sc和Zr含量较高。EDX分析结果表明该相中的化学配比为Al3(Sc,Zr)(Al和Sc+Zr的摩尔比为3?1)。从图1(b)可以看出,Al3(Sc,Zr)相占有最大体积分数。另外,衍射图谱中L12相的衍射强度明显高于D023相的,表明Al3(Sc,Zr)相与Al3Sc相具有相同的L12结构。据此可以认为Al3(Sc,Zr)相是Zr在Al3Sc相中的固溶体。
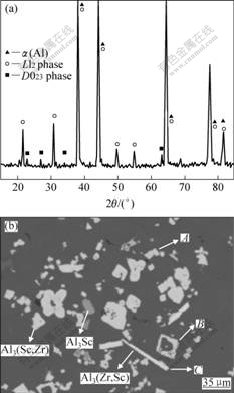
图1 合金1中初生相的XRD谱和形貌
Fig.1 XRD pattern (a) and morphology (b) of primary precipitates in alloy 1
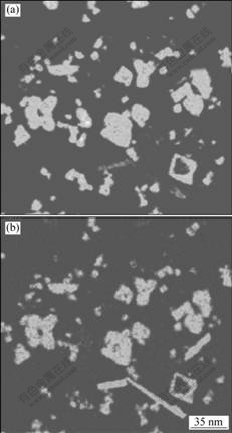
图2 合金1中初生相的EPMA结果
Fig.2 EPMA results of Sc (a) and Zr (b) mappings of primary precipitates in alloy 1
图3所示为过腐蚀处理后获得的初生相的表面形貌。由图3可看出,由于初生相的电位相对较高,在腐蚀过程中,周围的α(Al)基体受到侵蚀从而显露出初生相的表面形貌。在较慢的冷却速率下,合金1中初生相比较粗大。在图3(a)中可以看到,由若干连在一起的小相颗粒组成的初生相团簇,这种形貌非常类似于过共晶Al-Sc合金中的Al3Sc相团簇[3]。这种团簇状形貌是由于初生相在较小的冷却速率下形核较难引起的,在金相观察时会呈现出各种复杂形貌(见图1(b)中A)。在图3(b)可看到一个内部为空腔的Al3(Sc,Zr)相颗粒。该颗粒具有规则的立方外形,金相形貌为包含有α(Al)的环形(见图1(b)中B)。颗粒内部的空腔是α(Al)被腐蚀掉后产生的,表明该颗粒具有一种内部为α(Al)外层为Al3(Sc,Zr)相的复合结构。一般认为在凝固过程中初生相先于α(Al)基体生成[2, 13],然而,这种复合结构证明在较小的冷却速率下初生相也可以在已经结晶的α(Al)表面形核并长大。图3(c)所示为片状Al3(Zr,Sc)初生相的形貌,其金相形貌为图1(b)中C所示的长方条形。从图1(b)中可以发现一些较小的Al3(Sc,Zr)初生相附着在其表面。这可能是由于Al3(Zr, Sc) 相的形成温度高于Al3(Sc, Zr)相的所致。
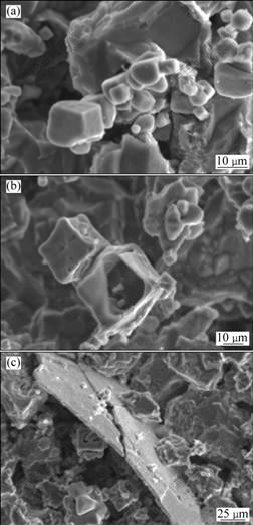
图3 图1(b)中初生相的表面形貌
Fig.3 Surface morphologies of precipitates A (a), B (b) and C (c) marked in Fig.1(b)
图4所示为钢模冷却Al-6Mg-0.2Sc-0.15Zr合金中初生相的XRD谱。从图4中的XRD谱中可以看出,合金2中初生相的体积分数明显低于合金1的,且只有L12结构的初生相生成。说明在冷却速率增大的情况下,D023相的析出受到抑制。EDX结果表明,合金2中的初生相具有Al3(Sc, Zr)的成分,Zr在(Sc+Zr)中的含量为50%~60%(质量分数)。虽然D023结构的Al3(Zr, Sc)相的形成受到了抑制,但仍然有可能生成L12 结构的Al3(Zr, Sc)相。在较大的冷却速率下,二元合金中具有L12结构的Al3X相(X=Zr,Hf)会代替平衡态的初生相生成[12, 14-15]。在钢模冷却的合金中,如果Al3Zr相以亚稳态的形式析出,Sc原子溶解在Al3Zr中将形成L12结构的Al3(Zr,Sc)相。这种具有L12结构的Al3(Zr,Sc)相是一种非平衡的化合物。亚稳态的Al3(Zr,Sc)相和Al3(Sc,Zr)相具有相同的晶体结构并且晶格常数非常接近,因此不能排除生成前者的可能。由图4可看出,虽然合金2中初生相总的质量分数低于合金1的,但合金2中的初生相具有更多的颗粒数量。
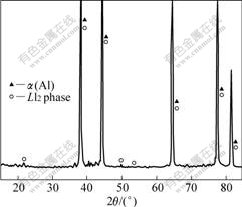
图4 钢模冷却Al-6Mg-0.2Sc-0.15Zr合金中初生相的XRD谱
Fig.4 XRD pattern of primary precipitates in Al-6Mg-0.2Sc- 0.15Zr alloy with steel mould
图5所示为合金2中初生相的截面形貌和表面形貌。由图5可看出,在晶粒内和晶界处存在尺寸小于10 μm的初生相颗粒。绝大多数Al3(Sc,Zr)相具有图5(b)所示的立方外形。当沿不同晶面截取时,则会出现正方、长方或三角等形貌。

图5 钢模冷却Al-6Mg-0.2Sc-0.15Zr合金中初生相截面和表面的形貌
Fig.5 Morphologies of section (a) and surface (b) of primary precipitates in Al-6Mg-0.2Sc- 0.15Zr alloy with steel mould
图6所示为用铜模具凝固得到的Al-6Mg-0.2Sc- 0.15Zr合金的铸态显微组织。由图6可看出,用铜模具冷却的试样中没有发现初生相的存在。合金3中晶粒尺寸大约为150 μm,明显大于合金2中50 μm的水平。在同样的条件下,合金3获得更大的过冷度。本应该产生更小的晶粒尺寸,但由于充当α(Al)形核中心的Al3(Sc, Zr)初生相的形成受到抑制,合金3的晶粒尺寸反而较为粗大。通常情况下,初生相会在α(Al)的结晶之前析出成为后者的形核中心。但当冷却速率增大到一定水平时,较大的过冷度使α(Al)快速降温并结晶。从热力学的角度来看初生相的形成有利于体系自由能的降低。但是,这个过程也是Sc和Zr原子扩散的过程,需要满足一定的动力学条件。当液态金属的冷却速率过快时,液态金属中的Sc和Zr原子来不及扩散就被固溶在固态的α(Al)中,从而抑制初生相的形成。
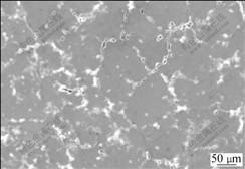
图6 铜快速冷却Al-6Mg-0.2Sc-0.15Zr合金的铸态显微组织
Fig.6 As-casting microstructure of rapidly cooled Al-6Mg- 0.2Sc-0.15Zr alloy with copper mould
在合金的凝固过程中,液态金属的冷却速率同时影响初生相和α(Al)基体的形核和长大过程。在合金1中,由于冷却速率较小,Al3(Sc, Zr)和Al3(Zr, Sc)相在α(Al)基体开始凝固前形成并生长至较大尺寸(见图3)。在合金2中,较大的冷却速率一方面增加初生相颗粒的形核数量,另一方面也增大α(Al)基体的过冷度。初生相长大至一定尺寸后,由于α(Al)在颗粒表面形核而停止生长。因此,合金2中初生相的尺寸较小(见图5(a))。在合金3中,液态金属的温度迅速下降至α(Al)的液相温度以下,在强烈的过冷作用下,α(Al)快速形核长大,从而抑制初生相的生成。
上述实验结果表明,液态金属的冷却速率能够对初生相的析出行为产生强烈的影响。随着冷却速率的加快,Al-6Mg-0.2Sc-0.15Zr合金中初生相的数量减少甚至消失。当初生相的析出受到抑制后,相应的Sc和Zr原子进入过饱和的α(Al)固溶体中;在随后的冷却和热处理过程中,将会从过饱和固溶体中析出更多的Al3(Sc, Zr)沉淀相颗粒,从而提高合金的强度和冷变形后的再结晶温度[16-17]。
3 结论
1) 在较低的冷却速率下(随炉冷却),初生相为L12结构的Al3(Sc, Zr)相和D023结构的Al3(Zr, Sc)相,随炉冷却的合金中初生相具有复杂的形貌和较大的 尺寸。
2) 在较大的冷却速率下(钢模冷却),D023结构的Al3(Zr, Sc)相的析出受到抑制而生成L12结构的Al3(Sc, Zr)相或亚稳态的Al3(Zr, Sc)相。钢模冷却的合金中初生相的体积分数较少,但形成的颗粒数量很多。
3) 快速冷却时(铜模冷却),α(Al)在初生相形成之前快速形核长大,初生相的析出完全受到抑制。
REFERENCES
[1] GSCHNEIDNER K A, CALDERWOOD F W. The Al-Sc (aluminum-scandium)system[J]. Bulletin of Alloy Phase Diagrams, 1989, 10(1): 34-36.
[2] NORMAN A F, PRANGNELL P B, MCEWEN R S. The solidification behaviour of dilute aluminium-scandium alloys[J]. Acta Materialia, 1998, 46(16): 5715-5732.
[3] HYDE K B, NORMAN A F, PRANGNELL P B. The effect of cooling rate on the morphology of primary Al3Sc intermetallic particles in Al-Sc alloys[J]. Acta Materialia, 2001, 49(8): 1327-1337.
[4] LIU Z X, LI Z J, WANG M X, WENG Y G. Effect of complex alloying of Sc, Zr and Ti on the microstructure and mechanical properties of Al-5Mg alloys[J]. Mater Sci Eng A, 2008, 483/484: 120-122.
[5] ZENG F H, XIA C Q, GU Y. The 430℃ isothermal section of the Al-4Mg-Sc-Zr quaternary system in the Al-rich range[J]. Journal of Alloys and Compounds, 2004, 363(1/2): 175-181.
[6] YIN Z M, PAN Q L, ZHANG Y H, JIANG F. Effect of minor Sc and Zr on the microstructure and mechanical properties of Al-Mg based alloys[J]. Mater Sci Eng A, 2000, 280: 151-121.
[7] SEIDMAN D N, MARQUIS E A, DUNAND D C. Precipitation strengthening at ambient and elevated temperatures of heat-treatable Al(Sc) alloys[J]. Acta Materialia, 2002, 50(16): 4021-4035.
[8] KARNESKY R A, MENG L, DUNAND D C. Strengthening mechanisms in aluminum containing coherent Al3Sc precipitates and incoherent Al2O3 dispersoids[J]. Acta Materialia, 2007, 55(4): 1299-1308.
[9] LIU F C, MA Z Y. Achieving exceptionally high superplasticity at high strain rates in a micrograined Al-Mg-Sc alloy produced by friction stir processing[J]. Scripta Materialia, 2008, 59(8): 882-885.
[10] CAVANAUGH M K, BIRDILIS N, BUCHHEIT R G, BOVARD F. Investigating localized corrosion susceptibility arising from Sc containing intermetallic Al3Sc in high strength Al-alloys[J]. Scripta Materialia, 2007, 56(11): 995-998.
[11] KENDIG K L, MIRACLE D B. Strengthening mechanisms of an Al-Mg-Sc-Zr alloy[J]. Acta Materialia, 2002, 50(16): 4165-4175.
[12] HORI S, SAJI S, TAKEHARA A. Structure of rapidly solidified Al-Zr alloys and its thermal stability[C]//MASUMOTO T, SUZUKI K. Proceeding of 4th International Conference on Rapidly Quenched Metals. Tokyo: Japan Institute of Metals, 1981: 1545-1548.
[13] DAVYDOV V G, ROSTOVA T D, ZAKHAROV V V, FILATOV Y A, YELAGIN V I. Scientific principles of making an alloying addition of scandium to aluminium alloys[J]. Mater Sci Eng A, 2000, 280(1): 30-36.
[14] HORI S, UNIGAME Y, FURUSHIRO N, TAI H. Phase decomposition in splat quenched Al-6% Hf alloy[J]. Journal of the Japan Institute of Light Metals, 1982, 32: 408-412.
[15] NORMAN A F, TSAKIROPOULOS P. Rapid solidification of Al-Hf alloys: Solidification microstructures and decomposition of solid solutions[J]. International Journal of Rapid Solidification, 1991, 6(3): 185-213.
[16] JONES M J, HUMPHREYS F J. Interaction of recrystallization and precipitation: The effect of Al3Sc on the recrystallization behaviour of deformed aluminium[J]. Acta Materialia, 2003, 51(8): 2149-2159.
[17] APPS P J , BERTA M, PRANGNELL P B. The effect of dispersoids on the grain refinement mechanisms during deformation of aluminium alloys to ultra-high strains[J]. Acta Materialia, 2005, 53(2): 499-511.
____________________________
收稿日期:2009-11-25;修订日期:2010-01-05
通信作者:戎利建,研究员,博士;电话:024-23971979;E-mail:ljrong@imr.ac.cn
(编辑 李艳红)


