
Effect of preparation conditions on selective oxidation of propane to acrylic acid
YU Zhen-xing(于振兴), ZHENG Wei(郑 伟), XU Wen-long(徐文龙),
ZHANG Yu-hang(张宇航), FU Hong-ying(付红英), ZHANG Ping(张 平)
Daqing Nanyuan Group Co., Ltd, Daqing 163517, China
Received 10 June 2009; accepted 15 August 2009
Abstract:
The effects of chemical composition and preparation conditions, especially calcination atmosphere and water content on the catalytic performances of MoVTeNbO mixed oxide catalyst system for the selective oxidation of propane to acrylic acid were investigated. Among the catalysts studied, Mo1.0V0.3Te0.23Nb0.12Ox catalyst calcined in inert atmosphere at 600 ℃ shows the best performance in terms of propane conversion and selectivity to acrylic acid. The results reveal that proper chemical composition, calcination atmosphere and water content affect greatly the catalysts in many ways including structure, chemical composition, which are related to their catalytic performances; and 51.0% propane conversion and 30.5% one-pass yield to acrylic acid can be achieved at the same time.
Key words:
selective oxidation; propane oxidation; catalysts preparation; acrylic acid; propane;
1 Introduction
Since the last decade, there has been a great interest in the development of highly active and selective catalysts for selective oxidation of light alkanes due to their potential application as a source of cheap raw materials in the petroleum and petrochemical industries [1]. Selective oxidation of propane is highly attractive because it would bypass the energy intensive endothermic steam cracking or dehydrogenation reactions currently employed to manufacture olefin intermediates from natural gas and petroleum feedstocks for subsequent oxidation. Catalytic selective oxidation of propane is motivated by both the potential economic and environmental advantages. It has recently attracted great attention in both academia and industry. Up to now, heteropolyacids and their derived salts[2], vanadyl pyrophosphate[3] and mixed metal oxides catalysts[4], have been studied for the acrylic acid production from propane. The most promising mixed metal oxide for propane selective oxidation is the bulk mixed Mo-V-Te-Nb-O system which was disclosed in recent years as highly active and selective catalyst for the ammoxidation of propane to acrylonitrile[5] and the propane oxidation to acrylic acid[6].
TU et al[7] reported bulk mixed Mo-V-Te-Nb-O system, which exhibited a 48% acrylic acid yield and 63.4% propane conversion at a reaction temperature of 380 ℃. Despite the promise of the results obtained, few papers have reported about the fundamental information. The information is very important for the development of this promising catalytic system for selective propane oxidation. In this work, the effects of metal molar ratio, calcination temperature, calcination atmosphere, water content and oxygen content on the catalytic performance were studied for the selective oxidation of propane into acrylic acid.
2 Experimental
2.1 Catalyst preparation
Source chemicals for the precursor compounds of the oxides were NH4VO3 for V, (NH4)6Mo7O24?4H2O for Mo and Te(OH)6 for Te, respectively. The precursor compound as the source for Nb was niobium oxalate. The desired amounts of ammonium metavanadate, ammonium heptamolybdate, and telluric acid were dissolved in deionized water and stirred at 80 ℃ for 1 h in a flask, resulting in a uniform aqueous solution. The solution was cooled to 40 ℃, and then an aqueous solution of niobium oxalate having the desired niobium concentration was mixed to obtain a slurry. The water of the slurry was removed via a rotavapor with a warm water bath at 60 ℃ at a reduced pressure of 1.33-5.32 kPa to obtain a dry powder precursor. The catalyst powder precursor was calcined at 400-750 ℃ for 4 h with a steady nitrogen stream in a covered tubular furnace so as to prevent the entry of air into the flask. The furnace had previously been heated to 200 ℃ at a rate of 2 ℃/min and held for 1 h, then ramped to 400- 750 ℃ at a rate of 2 ℃/min and held at that temperature for 2 h. After being cooled to room temperature, the resulting mixed metal oxide catalyst was obtained. Then the material was ground, pressed and sieved to granules with size of 0.85-1.70 nm.
2.2 Catalyst evaluation
The catalytic performance of the mixed metal oxide catalysts was evaluated for the selective oxidation of propane to acrylic acid in a continuous flow fixed bed micro-reactor testing at atmospheric pressure. About 8.0 g of granules catalyst was packed into a d 2 mm quartz tube in a programmable oven and heated in a chamber at a temperature of 400 ℃. A mixture of propane- oxygen-nitrogen-water vapour was fed in from the top of the reactor. The off-gas was condensed and separated from the liquid phase in several cold traps. Product streams were then analyzed by GC to determine the propane conversion and oxidation product distribution. The carbon balance was always above 95%. The performance of the catalyst was measured by propane conversion, yields of acrylic acid or other products, and specific selectivity. The selectivity, conversion and yield expressed in molar percentage form were calculated on a base of propane.
3 Results and discussion
3.1 Influence of calcination temperature on catalytic performance
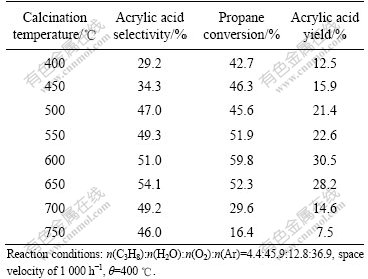
The calcination parameters, such as atmosphere, heating rate or temperature, can affect the resulting catalyst structures, which, in turn, affect the resulting catalytic performance in the selective oxidation of propane to acrylic acid[8]. The discussions here will be limited to only the effects of calcination temperatures. The catalysts precursor was calcined at different temperatures for 3 h. Results in Table 1 show that the reductions in either propane conversion and/or acrylic acid selectivity were observed even with minor changes in the calcination temperatures. It is clear that the calcination temperatures have strong effects on the products distribution. An increase in acrylic acid selectivity was observed with increasing temperature from 400 to 600 ℃. A further increase in temperature from 600 to 750 ℃ caused a decrease in the selectivity. It is well known from unsupported MoVTeNb-based catalysts that particular calcination temperatures have a crucial influence on phase composition and reduction degree of metal ions, which are related to the catalytic performance[9]. Among the mixed MoVTeNbO system catalysts prepared at different calcination temperatures, the highest propane conversion and acrylic acid selectivity are obtained at 600 ℃. So, it can be concluded that the catalytic activity for propane oxidation and the selectivity to acrylic acid depend strongly on the calcination temperatures.
Table 1 Effect of calcination temperature on catalytic performance
3.2 Influence of calcination atmosphere on catalytic performance
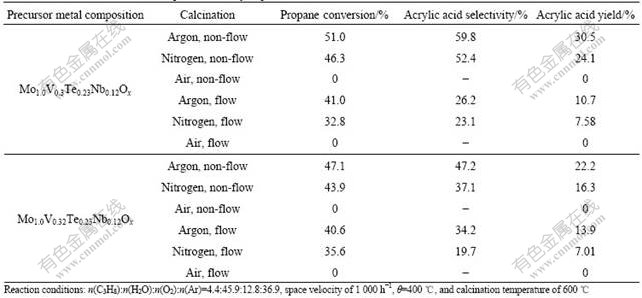
Calcination is typically the last step for the preparation of the mixed metal oxide catalyst materials. Many factors in the calcination step can affect the performance of the resulting catalysts. Two different catalyst precursors (Mo1.0V0.3Te0.23Nb0.12Ox and Mo1.0V0.32Te0.23Nb0.12Ox) were prepared with different metal molar ratio compositions. Each precursor was subjected to two different calcination atmospheres and flow conditions. The catalytic performances of these catalysts are summarized in Table 2.
Table 2 shows that as an inert gas with higher density than the air or the nitrogen, argon offers better blanketing effect than the nitrogen in an open calcination system to prevent air from contacting the catalysts in the bottom of calcination container. In the experimental set-up used in this study, the catalyst precursor was placed in the bottom of a container that has an opening to the atmosphere at the top which serves as the out-flow to prevent pressure build-up. On the other hand, the opening also makes it difficult to expel the air out completely. The use of the argon, which has a higher density than the air, in such a calcination set-up helps to exclude most of the air out, so providing an inert blanket covering the catalyst under calcination. On the whole, one does not expect the nitrogen to achieve the same blanketing effects as the argon does in the open system. Thus, our study leads to the conclusion that calcination under an inert atmosphere is preferred, and the argon is more preferred over the nitrogen.
Table 2 Effect of calcination atmosphere on catalytic performance
3.3 Influence of water content on catalytic perfor- mance
The presence of water vapour in the feed had a profound effect on the performance of mixed MoVTeNbO catalyst and the selectivity of propane oxidation to acrylic acid. The catalytic performance of the resulting mixed oxide systems is further found to be sensitive to the water content in Fig.1. The effect of water vapor was studied at 400 ℃ employing the mixed MoVTeNbO oxide catalyst and the feed containing 4.4:12.8:36.9 (molar ratio) of propane to oxygen to nitrogen and 30%-61% water vapor as a function of time on stream. The results shown in Fig.1 indicate that the selectivity to acrylic acid continuously changes as a function of water content in the feed. The activities of catalyst and reactor selectivity to acrylic acid are enhanced by the addition of water vapour. Both the acrylic acid yield and the selectivity to acrylic acid increase with the addition of 45.9% water vapour to achieve the maximum. It was found that the excess water vapour (more than 45.9%) inhibited the reaction, leading to acrylic acid probably due to an overadsorption on the catalyst surface.
These observations indicate that water has two distinct effects on propane oxidation over MoVTeNbO system catalysts. On one hand, the water enhances the rate of propane oxidation to acrylic acid. Probably, water content increases the concentration of hydroxyl groups on the catalyst surface and facilitates the reaction between the adsorbed acryloyl species and hydroxyl groups to form acrylic acid[10]. Water is adsorbed preferentially on Lewis acid sites to give Bronsted acid sites. As a consequence, the strongest adsorbent sites, where carboxylic oxygen bonds to the cation leading to deep oxidation of the reaction intermediates, are removed from catalyst surface[11], thus, enhancing the selectivity of the process and allowing the reoxidation of the catalyst surface[12]. On the other hand, water plays a very critical role in determining the desorption of the product, which generally possesses an acid function on the catalyst surface to prevent it from overoxidation to COx in the presence of water vapour. According to our results, water vapour may also take away the heat produced during the reaction to avoid the hot area on the catalyst surface.
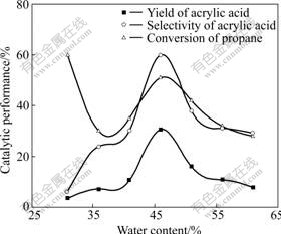
Fig.1 Effect of water content on catalytic performance under reaction conditions: n(C3H8)?n(O2)? n(Ar)=4.4?12.8?36.9, space velocity of 1 000 h-1, θ =400 ℃
3.4 Influence of metal molar ratios on catalytic per- formance
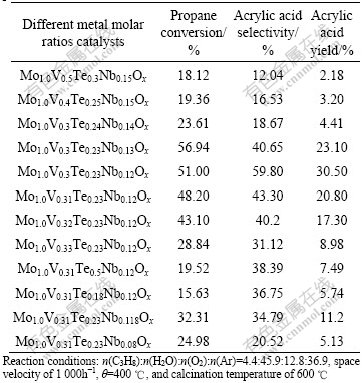
The catalytic performance of the resulting mixed oxides is further found to be sensitive to the relative metal ratios of the oxides. Results in Table 3 show that the preferred metal ratio is around the vicinity of Mo1.0V0.30Te0.23Nb0.12Ox. Significant reductions in either propane conversion or acrylic acid selectivity are observed even with minor changes in the metal ratio. Drastic reductions in catalytic activity and acrylic acid selectivity are observed when the metal molar ratios, especially those of Te, V and Nb further deviated away from the preferred range. The highest selectivities to acrylic acid were achieved on samples with different molar ratio in Table 3. In this way, it can be noticed that a high yield of acrylic acid of about 30.5% has been obtained at 400 ℃ and a propane conversion of about 51.0% on this optimal complex metal oxide catalyst.
Table 3 Effect of calcination temperatures on catalytic performance
4 Conclusions
1) The effect of water vapour on the selectivity of propane oxidation to acrylic acid is important. Both the catalytic activity for propane conversion and the selectivity to acrylic acid seem to be dramatically affected by the water vapour. The main role of steam is to enhance the desorption of acrylic acid from the catalyst surface to prevent it from overoxidation to catalyst under the optimized reaction conditions was examined.
2) The calcination temperature and calcination atmosphere can affect the resulting catalyst structures, which, in turn, affect the resulting catalytic performance in the selective oxidation of propane to acrylic acid.
3) Research in exploring an optimal ratio of metal offers a good opportunity to further improve the effectiveness of MoVTeNb complex oxides as catalysts for propane selective oxidation to acrylic acid.
References
[1] BEATO P, BLUME A, GIRGSDIES E, JENTOFT R E, SCHLOGEL R, TIMPE O, TRUNSCHKE A, WEINBERG G, BASHER Q, HAMID F A. Analysis of structural transformations during the synthesis of a MoVTeNb mixed oxide catalyst [J]. Applied Catalysis A: General, 2006, 307(1): 137-147.
[2] POPOVA G Y, ANDRUSHKEVICH T V, CHESALOV Y, PLYASOVA L M, DOVLITOVA L S, ISCHENKO E V, ALESHINA G L, KHRAMOV M I. Formation of active phases in MoVTeNb oxide catalysts for ammoxidation of propane [J]. Catalysis Today, 2009, 144(3/4): 312-317.
[3] YI Xiao-dong, SUN Xiao-dan, ZHANG Xiao-bing, HUANG Chuan-jing, WENG Wei-zheng, WAN Hui-lin. Highly dispersed MoVTeNbO/SiO2 catalysts prepared by the sol-gel method for selective oxidation of propane to acrolein [J]. Catalysis Communications, 2009, 10(12): 1591-1594.
[4] DENIAU B, MILLET J M, LORIDANT S, CHRISTIN N, DUBOIS J L. Effect of several cationic substitutions in the M1 active phase of the MoVTeNbO catalysts used for the oxidation of propane to acrylic acid [J]. Journal of Catalysis, 2008, 260(1): 30-36.
[5] DENG Zhong-hua, WANG Hong-xin, CHU Wen-ling, YANG Wei-shen. Influence of the reducing atmosphere on the structure and activity of Mo-V-Te-Nb-O catalysts for propane selective oxidation [J]. Chinese Journal of Catalysis, 2008, 29(10): 1032-1036.
[6] OH K S, WOO S I. Effect of preparation and reaction condition on the catalytic performance of Mo-V-Te-Nb catalysts for selective oxidation of propane to acrylic acid by high-throughput methodology [J]. Catalysis Today, 2008, 137(1): 61-70.
[7] TU X L, FURUTA N, SUMIDA Y, TAKAHASHI M, NIDUMA H. A new approach to the preparation of MoVNbTe mixed oxide catalysts for the oxidation of propane to acrylic acid [J]. Catalysis Today, 2006, 117(1/3): 259-264.
[8] MANHUA M L. Complex metal-oxide catalysts for selective oxidation of propane and derivatives (I): Catalysts preparation and application in propane selective oxidation to acrylic acid [J]. Applied Catalysis A: General, 2003, 250(2): 305-318.
[9] ZHENG W, YU Z X, ZHANG P, ZHANG Y H, FU H Y, ZHANG X L, SUN Q, HU X G. Selective oxidation of propane to acrylic acid over mixed metal oxide catalysts [J]. Journal of Natural Gas Chemistry, 2008, 17(2): 191-194.
[10] BALCELLS E, BORGMEIER F, GRI?TEDE I, LINTZH H G, ROSOWSKI F. Partial oxidation of propane to acrylic acid at a Mo-V-Te-Nb-oxide catalyst [J]. Applied Catalysis A: General, 2004, 266(2): 211-221.
[11] MIN J E M, MIZUNO M. Iron as an effective additive for enhancement of catalytic performance of cesium hydrogen salt of molybdophosphoric acid for selective oxidation of isobutane, propane, and ethane under oxygen-rich and -poor conditions and the catalyst design [J]. Catalysis Today, 2001, 66(1): 47-52.
[12] HIBST H, ROSOWSKI F, COX G. New Cs-containing Mo-V4+ based oxides with the structure of the M1 phase-base for new catalysts for the direct alkane activation [J]. Catalysis Today, 2006, 117(1/3): 234-241.
(Edited by YANG Hua)
Corresponding author: YU Zhen-xing; Tel: +86-459-4494766; Fax: +86-459-4498336; E-maill: yuzxing@sohu.com


