
Calculating models of mass action concentrations for structural units or
ion couples in RbCl-H2O binary system and RbCl-RbNO3-H2O ternary system
GUO Han-jie(郭汉杰)1, YANG Xue-min(杨学民)2, ZHAO Wei-jie(赵伟洁)1
1. School of Metallurgical and Ecological Engineering,
University of Science and Technology Beijing, Beijing 100083, China;
2. State Key Laboratory of Multiphase Complex System, Institute of Process Engineering,
Chinese Academy of Sciences, Beijing 100190, China
Received 6 July 2009; accepted 4 January 2010
Abstract:
Thermodynamic models of calculating mass action concentrations for structural units or ion couples in RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions were developed based on the ion and molecule coexistence theory at 298.15 K. A transformation coefficient is needed to compare the calculated mass action concentration and the reported activity because they are obtained at different standard states and concentration units. The results show that the transformation coefficients between the calculated mass action concentrations and the reported activities of the same structural units or ion couples in RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions change in a very narrow range. The transformed mass action concentrations of structural units or ion couples in RbCl-H2O binary system are in good agreement with the reported activities. The transformed mass action concentrations of RbCl and RbNO3 in RbCl-RbNO3-H2O ternary solution are also in good agreement with the reported activities, aRbCl and ![]() , with different total ionic strengths as 0.01, 0.05, 0.1, 0.5, 1.0, 1.5, 2.0, 3.0 and 3.5 mol/kg, respectively. All those results mean the developed thermodynamic model of strong electrolyte aqueous solutions can reflect structural characteristics of RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions and the mass action concentration also strictly follows the mass action law.
, with different total ionic strengths as 0.01, 0.05, 0.1, 0.5, 1.0, 1.5, 2.0, 3.0 and 3.5 mol/kg, respectively. All those results mean the developed thermodynamic model of strong electrolyte aqueous solutions can reflect structural characteristics of RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions and the mass action concentration also strictly follows the mass action law.
Key words:
mass action concentration; activity; ion and molecule coexistence theory; RbCl-H2O; RbCl-RbNO3-H2O; structural unit; ion coupl;
1 Introduction
The ion and molecule coexistence theory developed by ZHANG[1-8] since 1980s has been successfully applied to calculate mass action concentrations of structural units or ion couples in binary metallic melts[1], binary metallurgical slags[2], ternary metallic melts[3-4], ternary metallurgical slags[5-6] and multi-component complex metallurgical slags[7-8]. Expanding application scopes of the ion and molecule coexistence theory to electrolyte aqueous solutions is an interesting and challenge task. It is well-known that electrolyte aqueous solutions can be applied in many fields, such as biochemical engineering[9-12], chemical engineering [13], hydrometallurgy, environmental chemistry, and geochemistry[14]. However, activity of components in electrolyte aqueous solutions, as one of main thermodynamic properties, is not enough, or scarce and contradictory among literatures, or imprecise to fulfill its practical applications.
It has been well-known that not only ions but also some simple or complex molecules can exist in electrolyte aqueous solutions. Therefore, the ion and molecule coexistence theory, which is developed to determine mass action concentration of structural units or ion couples to present reaction ability like activity, can be applied to describe reaction ability of electrolyte aqueous solutions.
Based on the successful applications of the ion and molecule coexistence theory for calculating mass action concentrations of structural units or ion couples in KCl-H2O, CsCl-H2O, NaCl-H2O, BaCl2-H2O[15], NaBr-H2O, LiNO3-H2O, HNO3-H2O, KF-H2O binary strong electrolyte aqueous solutions[16], and NaCl-KCl- H2O[17], it can be generally deduced that the calculated mass action concentrations of structural units or ion couples have close corresponding relation with the reported activities for strong electrolyte aqueous solutions. Therefore, the calculated mass action concentrations of structural units or ion couples from the ion and molecular coexistence theory can be effectively applied to present reaction ability of components in binary and ternary strong electrolyte aqueous solutions.
To further extend application scopes of the ion and molecule coexistence theory into multi-component strong electrolyte aqueous solutions, a thermodynamic model of calculating mass action concentrations for structural units or ion couples in RbCl-RbNO3-H2O ternary strong electrolyte aqueous solution has been proposed according to the developed universal thermodynamic model of calculating mass action concentrations for structural units or ion couples in ternary strong electrolyte aqueous solutions[15]. To clearly describe the proposed thermodynamic model of calculating mass action concentrations for structural units or ion couples in RbCl-RbNO3-H2O ternary aqueous solution, a thermodynamic model of calculating mass action concentrations for structural units or ion couples in RbCl-H2O binary strong electrolyte aqueous solutions is also proposed by applying the developed thermodynamic model of calculating mass action concentrations for structural units or ion couples in binary strong electrolyte aqueous solutions reported elsewhere[15-17].
The calculated mass action concentrations of RbCl and RbNO3 have been compared with the reported activities[18] with different total ionic strengths as 0.01, 0.05, 0.1, 0.5, 1.0, 1.5, 2.0, 3.0 and 3.5 mol/kg in RbCl-RbNO3-H2O ternary solution at 298.15 K. Because the calculated mass action concentrations are usually based on pure species as standard state and mole fraction as concentration unit while the reported activity is based on infinite dilution as standard state and molality as concentration unit, a transformation coefficient should be needed for comparing the calculated mass action concentrations with the reported ones.
2 Thermodynamic model of calculating mass action concentrations
2.1 Hypotheses
The main viewpoints of the ion and molecule coexistence theory for strong electrolyte aqueous solutions can be summarized as follows.
1) Strong electrolyte aqueous solutions are composed of Na+, Ca2+, Mg2+, Rb+, Cl-, Br-, F-, ![]() , etc, as simple ions, H2O as simple molecule, and hydrous salt compounds as complex molecules. It is assumed that each cation and anion of the simple ions occupies only one position of structural units, but will take part in the reaction of forming hydro salt molecules in the form of ion couple based on electrovalence balance. Choosing RbCl as an example, RbCl can be electrolyzed or separated into two simple ions as Rb+ and Cl-, respectively, as two structural units, but ions of Rb+ and Cl- will take part in the reaction of forming hydro salt molecules as an ion couple in form of (Rb++Cl-) in RbCl-contained aqueous solutions if there are hydro salt molecules formed.
, etc, as simple ions, H2O as simple molecule, and hydrous salt compounds as complex molecules. It is assumed that each cation and anion of the simple ions occupies only one position of structural units, but will take part in the reaction of forming hydro salt molecules in the form of ion couple based on electrovalence balance. Choosing RbCl as an example, RbCl can be electrolyzed or separated into two simple ions as Rb+ and Cl-, respectively, as two structural units, but ions of Rb+ and Cl- will take part in the reaction of forming hydro salt molecules as an ion couple in form of (Rb++Cl-) in RbCl-contained aqueous solutions if there are hydro salt molecules formed.
2) There are dynamic reaction equilibria of forming complex hydro salt molecules between ion couples and simple molecules of H2O.
3) The structural units in strong electrolyte aqueous solutions keep continuity in the investigated concentration range of compositions.
4) Chemical reactions of forming complex hydro slat molecules in strong electrolyte aqueous solution follow the mass action law.
2.2 Calculating model of mass action concentration for structural units or ion couples in RbCl-H2O binary system
2.2.1 Structural units and calculating model
It has been confirmed[19] that RbCl and H2O cannot form any hydrous salt molecule at 298.15 K. Hence, the structural units in RbCl-H2O binary solution are Rb+ and Cl- as ions and H2O as simple molecule. The molality mi (mol/kg) is usually applied to present the concentration of components in strong electrolyte aqueous solution. Therefore, the amounts-of-substance of RbCl and H2O in RbCl-H2O binary solution based on 1 kg H2O before dynamic reaction equilibrium is expressed as![]() ,
, ![]() 55.6 mol, respectively. The mole fraction of RbCl and H2O before dynamic equilibrium can be determined by
55.6 mol, respectively. The mole fraction of RbCl and H2O before dynamic equilibrium can be determined by ![]()
The equilibrium amount-of-substance of structural units in 1 kg H2O in RbCl-H2O binary solution is defined according to the ion and molecule coexistence theory as
![]() and
and ![]() =55.6
=55.6
mol. The total amount of substance of all structural units in 1 kg H2O in RbCl-H2O binary solution under equilibrium condition, ![]() , can be calculated according to mass balance principle as
, can be calculated according to mass balance principle as
![]() (1)
(1)
The mass action concentrations of each structural unit or ion couple, Ni, can be determined as
![]() (2a)
(2a)
![]() (2b)
(2b)
The mass balance of RbCl and H2O in 1 kg H2O in RbCl-H2O binary solution can be expressed as
![]() (3a)
(3a)
![]() (3b)
(3b)
The following equation can be obtained from the fact that the total equilibrium molar fraction of all structural units in a system is 1.0, that is
N1+N3=1 (4)
The Eqs.(3) and (4) are the calculating thermodynamic models of mass action concentration for structural units or ion couples in RbCl-H2O binary solution. There are three unknown parameters as N1, N3 and ![]() with three independent equations in the thermodynamic model composed of Eq.(3) and Eq.(4). Therefore, the real solutions of above-mentioned mass action concentration of structural units or ion couples, Ni, and total amount of substance of all structural units,
with three independent equations in the thermodynamic model composed of Eq.(3) and Eq.(4). Therefore, the real solutions of above-mentioned mass action concentration of structural units or ion couples, Ni, and total amount of substance of all structural units, ![]() , in RbCl-H2O binary solution can be solely solved by combining Eq.(3) and Eq.(4).
, in RbCl-H2O binary solution can be solely solved by combining Eq.(3) and Eq.(4).
The calculated mass action concentrations of solute, i.e., N1, is based on pure species as standard state and mole fraction, xi, as concentration unit; however, the reported activities are usually based on infinite dilution as standard state and molality mi as concentration unit. To compare the calculated mass action concentrations and the reported activities of solute, a transformation coefficient of RbCl, ![]() in RbCl-H2O binary solution is needed. The transformation coefficient,
in RbCl-H2O binary solution is needed. The transformation coefficient, ![]() and the transformed mass action concentration,
and the transformed mass action concentration, ![]() of RbCl in RbCl-H2O binary solution can be described as follows:
of RbCl in RbCl-H2O binary solution can be described as follows:
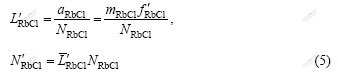
It should be specially pointed out that although no any hydro salt molecule can be formed in RbCl-H2O binary solution at 298.15 K, there are not independent RbCl molecules, but Rb+ and Cl- ions in RbCl-H2O binary solution. Using concept of mass action concentration for RbCl is just for convenience to compare the measured RbCl activity data with the calculated mass action concentrations of the same component because only activity data of two components in RbCl-H2O binary solution as RbCl and H2O can be determined and reported from viewpoints of classic experimental tests and traditional thermodynamics. The real meaning of mass action concentration for RbCl,![]() , is the sum of mass action concentration for two structural units in RbCl as Rb+ and Cl- in form of ion couple.
, is the sum of mass action concentration for two structural units in RbCl as Rb+ and Cl- in form of ion couple.
2.2.2 Results and discussion
The relationship between the transformed mass action concentration,![]() , or the reported activity, aRbCl[20], and mole fraction of RbCl, xRbCl, in RbCl-H2O binary solution at 298.15K is shown in Fig.1(a). Similarly, the calculated mass action concentration,
, or the reported activity, aRbCl[20], and mole fraction of RbCl, xRbCl, in RbCl-H2O binary solution at 298.15K is shown in Fig.1(a). Similarly, the calculated mass action concentration, ![]() , against
, against ![]() in RbCl-H2O binary solution is illustrated in Fig.1(b). It can be observed from Fig.1(a) that the transformed mass action concentration,
in RbCl-H2O binary solution is illustrated in Fig.1(b). It can be observed from Fig.1(a) that the transformed mass action concentration,![]() , is in good agreement with reported activity, aRbCl[21], in the investigated RbCl concentration range. The transformation coefficients
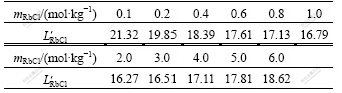
, is in good agreement with reported activity, aRbCl[21], in the investigated RbCl concentration range. The transformation coefficients ![]() between the transformed mass action concentrations and the reported activities of RbCl in RbCl-H2O binary solution are also listed in Table 1 in a molality range of RbCl from 0.1 to 6.0 mol/kg(H2O). It can be observed from Table 1 thatthe transformation coefficients of RbCl,
between the transformed mass action concentrations and the reported activities of RbCl in RbCl-H2O binary solution are also listed in Table 1 in a molality range of RbCl from 0.1 to 6.0 mol/kg(H2O). It can be observed from Table 1 thatthe transformation coefficients of RbCl,![]() , are more or less discrete, but an average datum of
, are more or less discrete, but an average datum of ![]() can be calculated to be 17.95. Therefore, it can be deduced that the calculated mass action concentrations can be applied to express reaction ability of RbCl and have a close relationship with the reported activities of RbCl in RbCl-H2O binary solution. All hypotheses used during the development of the thermodynamic model are reasonable and have got the facts of intrinsic structure of RbCl-H2O binary solution.
can be calculated to be 17.95. Therefore, it can be deduced that the calculated mass action concentrations can be applied to express reaction ability of RbCl and have a close relationship with the reported activities of RbCl in RbCl-H2O binary solution. All hypotheses used during the development of the thermodynamic model are reasonable and have got the facts of intrinsic structure of RbCl-H2O binary solution.
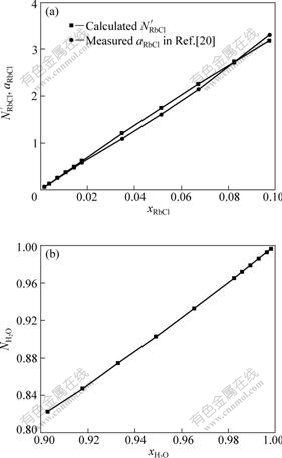
Fig.1 Comparison of transformed mass action concentrations![]() with reported activities
with reported activities ![]() of RbCl (a) and relationship between calculated mass action concentration
of RbCl (a) and relationship between calculated mass action concentration ![]() and mole fraction of H2O
and mole fraction of H2O ![]() (b) in RbCl-H2O binary solution at 298.15 K
(b) in RbCl-H2O binary solution at 298.15 K
Table 1 Transformation coefficients between calculated mass action concentrations and reported activities of RbCl in RbCl-H2O solutions
2.3 Calculating model of mass action concentration for structural units or ion couples in RbCl-RbNO3-H2O ternary system
2.3.1 Structural units and calculating model
It has been known[19] that RbCl and RbNO3 will be completely ionized and no hydrous salt molecules can be formed in RbCl-RbNO3-H2O ternary system at 298.15 K. Based on the ion and molecule coexistence theory, the structural units in RbCl-RbNO3-H2O ternary solution are composed of Rb+, Cl- and ![]() as simple ions, and H2O as simple molecule. According to above-mentioned hypotheses for strong electrolyte aqueous solution, the amount-of-substance of solutes and solvent in RbCl-RbNO3-H2O ternary solution based on 1 kg H2O before dynamic reaction equilibrium can be expressed as
as simple ions, and H2O as simple molecule. According to above-mentioned hypotheses for strong electrolyte aqueous solution, the amount-of-substance of solutes and solvent in RbCl-RbNO3-H2O ternary solution based on 1 kg H2O before dynamic reaction equilibrium can be expressed as![]()
![]() and
and ![]()
![]() respectively. The mole fraction of component i before equilibrium is presented as
respectively. The mole fraction of component i before equilibrium is presented as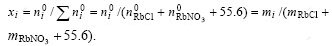
The equilibrium amount-of-substance of above- mentioned structural units in RbCl-RbNO3-H2O ternarysolution can be presented as ![]()
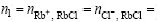
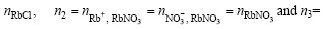 nH2O=55.6 mol. Hence, the total amount- of- substance of all structural units in 1 kg H2O in RbCl-RbNO3-H2O ternary solution under equilibrium,
nH2O=55.6 mol. Hence, the total amount- of- substance of all structural units in 1 kg H2O in RbCl-RbNO3-H2O ternary solution under equilibrium,![]() can be expressed according to mass balance principle as
can be expressed according to mass balance principle as
![]() (6)
(6)
The mass action concentrations of structural units or ion couples in RbCl-RbNO3-H2O ternary solution, i.e., RbCl, RbNO3 and H2O, are presented as
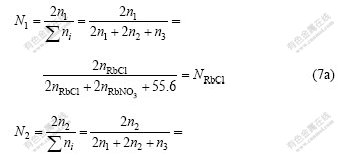
![]() (7b)
(7b)
![]()
![]() (7c)
(7c)
The mass balance of solutes and solvent in 1 kg H2O in RbCl-RbNO3-H2O ternary solution can be expressed, based on the ion and molecules coexistence theory, as
![]() (8a)
(8a)
![]() (8b)
(8b)
![]() (8c)
(8c)
The following equation can also be obtained from the fact that the total equilibrium molar fraction of all structural units in a system is 1.0, as
N1+N2+N3=1 (9)
The Eqs.(8) and (9) consist of the calculating thermodynamic model of mass action concentration for RbCl-RbNO3-H2O ternary solution. There are four unknown parameters as N1, N2, N3 and ![]() with four independent equations in the thermodynamic model composed of Eq.(8) and Eq.(9). The real solutions of above-mentioned mass action concentration of structural units or ion couples, Ni, and total amount of substance of
with four independent equations in the thermodynamic model composed of Eq.(8) and Eq.(9). The real solutions of above-mentioned mass action concentration of structural units or ion couples, Ni, and total amount of substance of
all structural units,![]() in RbCl-RbNO3-H2O ternary
in RbCl-RbNO3-H2O ternary
solution can be solely solved by combining Eq.(8) and Eq.(9).
To compare the calculated mass action concentrations and the reported activities of solutes, the transformation coefficients of RbCl, ![]() , and RbNO3,
, and RbNO3, ![]() , and the transformed mass action concentration,
, and the transformed mass action concentration, ![]() and
and ![]() , can be described as follows:
, can be described as follows:
![]() ,
,
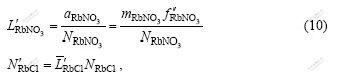
![]() (11)
(11)
2.3.2 Results and discussion
The activity coefficients of RbCl and RbNO3 in RbCl-RbNO3-H2O ternary solution have been reported elsewhere[18] in a range of total ionic strength, i.e., I=![]() +
+![]() , from 0.01 to 3.5 mol/kg with differentionic strength fraction of RbNO3,
, from 0.01 to 3.5 mol/kg with differentionic strength fraction of RbNO3, ![]()
![]() as 0, 0.2, 0.4, 0.6, and 0.8 at each total ionic strength, respectively. The transformed mass action concentrations,
as 0, 0.2, 0.4, 0.6, and 0.8 at each total ionic strength, respectively. The transformed mass action concentrations, ![]() and
and ![]() , have been compared with the reported activities[18], aRbCl and
, have been compared with the reported activities[18], aRbCl and ![]() , with different I, i.e., various mole fraction of RbCl, xRbCl, and RbNO3,
, with different I, i.e., various mole fraction of RbCl, xRbCl, and RbNO3, ![]() , as illustrated in Figs.2(a)-(r), respectively. It can be observed from Figs.2(a)-(r) that the transformed mass action concentrations,
, as illustrated in Figs.2(a)-(r), respectively. It can be observed from Figs.2(a)-(r) that the transformed mass action concentrations, ![]() and
and ![]() , are in
, are in
good agreement of the reported activities[18], ![]() and
and ![]() , in a large change range of I, i.e., with various xRbCl and
, in a large change range of I, i.e., with various xRbCl and ![]() respectively. The trans- formation coefficients,
respectively. The trans- formation coefficients, ![]() and
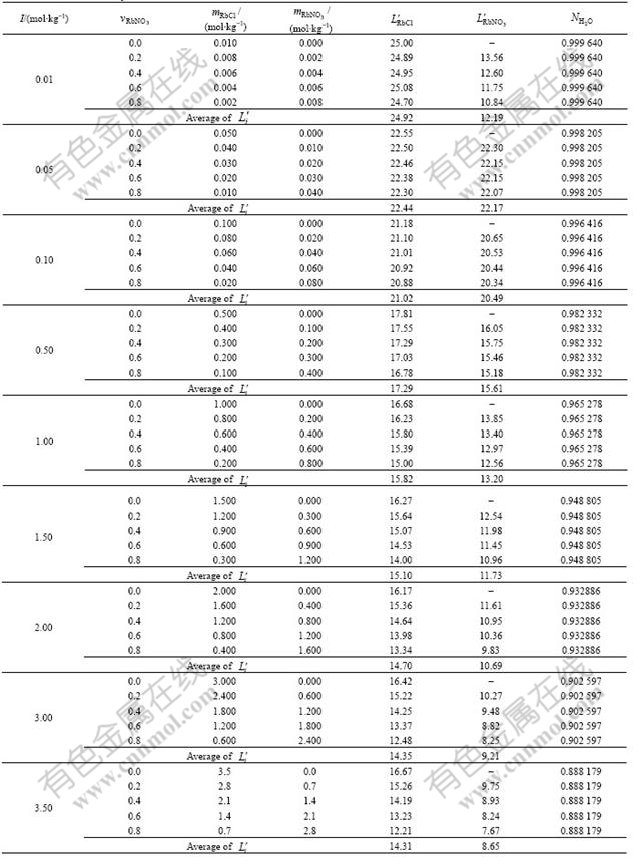
and ![]() , listed in Table 2, show that
, listed in Table 2, show that ![]() and
and ![]() keep constant with small deviations for different I, respectively. The calculated mass action concentrations of H2O,
keep constant with small deviations for different I, respectively. The calculated mass action concentrations of H2O, ![]() , are also summarized in Table 2 with different I. Hence, the ion and molecule coexistence theory can be successfully applied to calculate the reaction ability of components in RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions.
, are also summarized in Table 2 with different I. Hence, the ion and molecule coexistence theory can be successfully applied to calculate the reaction ability of components in RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions.
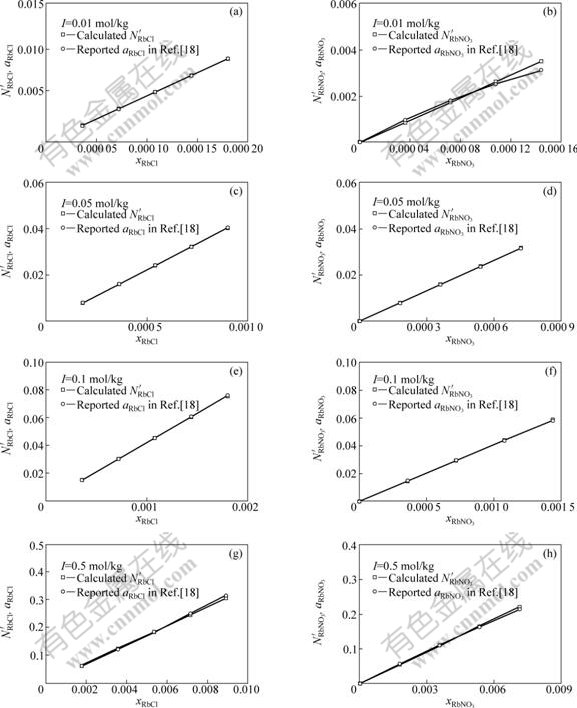
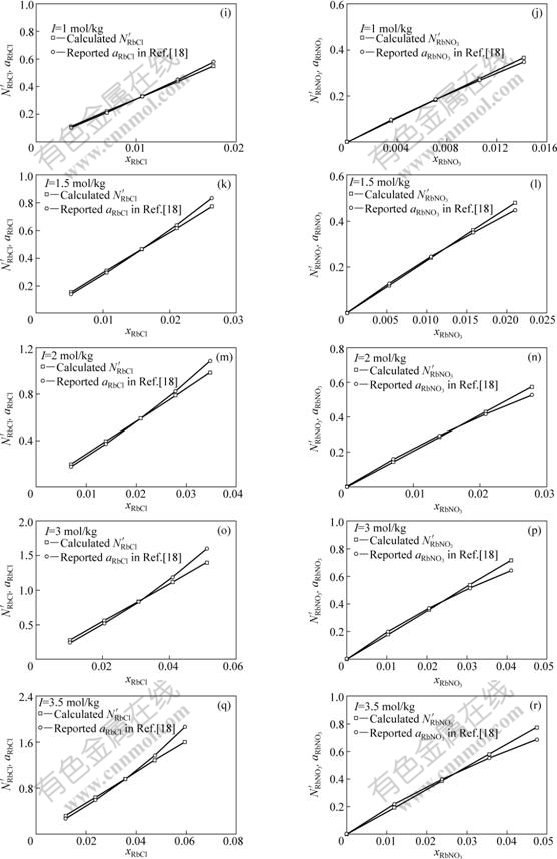
Fig.2 Comparison of transformed mass action concentrations,![]() and
and ![]() with reported activities, aRbCl and
with reported activities, aRbCl and ![]() , of RbCl and NbNO3 in RbCl-RbNO3-H2O ternary solution with total ionic strength I as 0.01, 0.05, 0.1, 0.5, 1.0, 1.5, 2.0, 3.0, and 3.5 mol/kg, respectively, at 298.15 K
, of RbCl and NbNO3 in RbCl-RbNO3-H2O ternary solution with total ionic strength I as 0.01, 0.05, 0.1, 0.5, 1.0, 1.5, 2.0, 3.0, and 3.5 mol/kg, respectively, at 298.15 K
Table 2 Transformation coefficients, ![]() , and
, and ![]() , in total ionic strength range from 0.01 to 3.5 mol/kg (H2O) with different ionic strengths of RbNO3,
, in total ionic strength range from 0.01 to 3.5 mol/kg (H2O) with different ionic strengths of RbNO3, ![]() , as 0, 0.2, 0.4, 0.5, 0.6 and 0.8, respectively, and calculated mass action concentration of H2O in RbCl-RbNO3-H2O ternary solution
, as 0, 0.2, 0.4, 0.5, 0.6 and 0.8, respectively, and calculated mass action concentration of H2O in RbCl-RbNO3-H2O ternary solution
The common ion in RbCl-RbNO3-H2O ternary system is Rb+, which can be generated from RbCl as well as RbNO3. However, the calculated mass action concentration of RbCl only considers the contribution of Rb+ and Cl- which exist in RbCl, but not the contribution of Rb+ in RbNO3. The same method is also used to calculate the mass action concentration of RbNO3. The treatment of the effect of common ion, i.e., Rb+ in RbCl-RbNO3-H2O ternary system, on calculation of mass action concentration for RbCl and RbNO3 needs further investigation in viewpoint of electrochemistry for strong electrolyte aqueous solutions.
The wonderful agreement between the transformed mass action concentrations and the reported activities suggests that the developed thermodynamic model can exactly reflect the structural characteristics of RbCl-RbNO3-H2O ternary solution and the mass action law is applicable to RbCl-RbNO3-H2O ternary strong electrolyte aqueous solution.
The calculated mass action concentrations of structural units or ion couples in RbCl-RbNO3-H2O ternary solution are valid by transformation coefficient, which need the reported activity data based on infinite dilution as standard state and molality as concentration unit. This maybe is the main reason for more or less deviation shown in Figs.2(a)-(r). The reasonable method is to compare the calculated mass action concentration of components and the measured activity with pure matter as standard state and mole fraction as concentration unit.
3 Conclusions
1) The calculated mass action concentrations of structural units or ion couples in RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions are in good agreement with the reported activities from literatures. Therefore, the developed thermodynamic model of calculating mass action concentrations for structural units or ion couples in RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions can be successfully applied to predict the reaction ability of components.
2) The ion and molecule coexistence theory in combination with the mass action law (if necessary when hydrous salt molecules as complex molecules exist) can be successfully applied to describe the structural characteristics of RbCl-H2O binary and RbCl-RbNO3- H2O ternary strong electrolyte aqueous solutions.
3) As there are visible deviations between the calculated mass action concentrations and the reported activities in high concentration range for two investigated aqueous solutions, it is absolutely necessary to carry out further investigation on reaction ability of structural units or ion couples in strong electrolyte aqueous solutions based on pure species as standard state and mole fraction as concentration unit.
Symbol list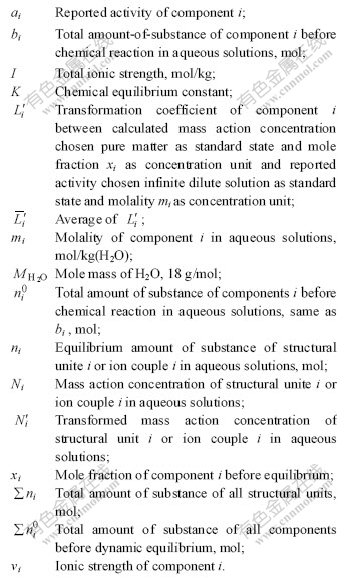
References
[1] ZHANG J. Calculation model of mass action concentrations for Mg-Al, Sr-Al and Ba-Al melts and determination of their thermodynamic parameters [J]. Journal of Iron and Steel Research International, 2003, 10(2): 5-9.
[2] ZHANG Jian. Calculation model of mass action concentrations CaO-SiO2 slag system [J]. Journal of University of Science and Technology Beijing, 1988,10(4): 412-421. (in Chinese)
[3] ZHANG J. Calculation model of mass action concentrations for Ag-Au-Cu melts [J]. Rare Metals, 2002, 21(1): 43-47.
[4] ZHANG J. Application of the annexation principle to the thermodynamic property study of ternary metallic melts In-Bi-Cu and In-Sb-Cu [J]. Journal of University of Science and Technology Beijing, 2002, 9(3): 170-176.
[5] ZHANG Jian, YUAN W X. Calculation model of mass action concentrations CaO-Al2O3-SiO2 slag system [J]. Journal of University of Science and Technology Beijing, 1995, 17(5): 418-423. (in Chinese)
[6] ZHANG Jian. Calculation model of mass action concentrations FeO-Fe2O3-SiO2 slag system [J]. Journal of University of Science and Technology Beijing, 1988, 10(1): 1-6. (in Chinese)
[7] ZHANG Jian. Manganese equilibrium between FeO-MnO-MgO- SiO2 slags and molten iron [J]. Journal of University of Science and Technology Beijing, 1992, 14(5): 496-501. (in Chinese)
[8] ZHANG J. Application of the coexistence theory of slag structure to multi-component slag systems [C]//Proc. 4th International Conference On Molten Slags and Fluxes. Sendai, Japan: The Iron and Steel Institute of Japan: 1992: 244-249.
[9] CHAN C K, HA Z Y, CHOI M Y. Study of water activities of aerosols of mixtures of sodium and magnesium salts [J]. Atmospheric Environment, 2000, 34(28): 4795-4803.
[10] HA Z Y, CHAN C K. The water activities of MgCl2, Mg(NO3)2, MgSO4, and their mixtures [J]. Aerosol Sci Technol, 1999, 31(2/3): 154-169.
[11] Pruppacher H R, Klett J D. Microphysics of clouds and precipitation [M]. Dordrecht, Holland: Reidel Publishing Company, 1978.
[12] Molina M J, Zhang R, Wooldridge P J, McMahon J R, KIM J E, CHANG H Y, BEYER K D. Physical chemistry of the H2SO4/HNO3/H2O system: Implications for polar stratospheric clouds [J]. Science, 1993, 261(5127): 1418-1423.
[13] Gokcen N A. Determination and estimation of ionic activities of metal salts in water. No.8372 [R]. Washington DC: Department of the Interior, Bureau of Mines, 1979.
[14] Guendouzi M E, Dinane A. Determination of water activities, osmotic and activity coefficients in aqueous solutions using the hygrometric method [J]. J Chem Thermodynamics, 2000, 32(3): 297-310.
[15] GUO Han-jie, Zhao Wei-jie, LI Lin, Yang Xue-min. A universal thermodynamic model of calculating mass action concentration of components in strong electrolyte binary aqueous solutions [J]. The Chinese Journal of Process Engineering, 2007, 7(2): 347-353. (in Chinese)
[16] GUO H J, Zhao W J, Yang X M. Calculating models of mass action concentrations for NaBr(aq), LiNO3(aq), HNO3(aq), and KF(aq) binary solutions [J]. Journal of University of Science and Technology Beijing, 2007, 14(3): 204-211.
[17] Zhao W J, GUO H J, Yang X M. A universal thermodynamic model of calculating mass action concentrations for components in ternary strong electrolyte aqueous solution and its application in NaCl-KCl-H2O system [J]. Journal of University of Science and Technology Beijing, 2008, 15(5): 543-551.
[18] Zhang J, Huang Y, Xia S P. Experimental determination and prediction of activity coefficients of RbCl in aqueous (RbCl+RbNO3) mixture at T=298.15 K [J]. The Journal of Chemical Thermodynamics, 2005, 37(11): 1162-1167.
[19] WAGMAN D D, EVANS W H, EVANS W H, PARKER V B, SCHUMM R H, HALOW I, BAILEY S M, CHURNEY K L, NUTTALL R L. The NBS tables of chemical thermodynamic properties-selected values for inorganic and C1 and C2 organic substances in SI units [M]. LIU Tian, ZHAO Meng-yue. Beijing: Chinese Standards Press, 1998. (in Chinese)
[20] Рабинович В А. Concise handbook of chemistry [M]. Beijing: Chemical Industry Press, 1983: 651-659. (in Chinese)
Foundation item: Project supported by Publication Foundation of National Science and Technology Academic Books of China
Corresponding author: YANG Xue-min; Tel: +86-10-82622893; Fax: 86-10-82622893; E-mail: yangxm71@home.ipe.ac.cn
DOI: 10.1016/S1003-6326(09)60265-X
(Edited by YANG Bing)
Abstract: Thermodynamic models of calculating mass action concentrations for structural units or ion couples in RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions were developed based on the ion and molecule coexistence theory at 298.15 K. A transformation coefficient is needed to compare the calculated mass action concentration and the reported activity because they are obtained at different standard states and concentration units. The results show that the transformation coefficients between the calculated mass action concentrations and the reported activities of the same structural units or ion couples in RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions change in a very narrow range. The transformed mass action concentrations of structural units or ion couples in RbCl-H2O binary system are in good agreement with the reported activities. The transformed mass action concentrations of RbCl and RbNO3 in RbCl-RbNO3-H2O ternary solution are also in good agreement with the reported activities, aRbCl and ![]() , with different total ionic strengths as 0.01, 0.05, 0.1, 0.5, 1.0, 1.5, 2.0, 3.0 and 3.5 mol/kg, respectively. All those results mean the developed thermodynamic model of strong electrolyte aqueous solutions can reflect structural characteristics of RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions and the mass action concentration also strictly follows the mass action law.
, with different total ionic strengths as 0.01, 0.05, 0.1, 0.5, 1.0, 1.5, 2.0, 3.0 and 3.5 mol/kg, respectively. All those results mean the developed thermodynamic model of strong electrolyte aqueous solutions can reflect structural characteristics of RbCl-H2O binary and RbCl-RbNO3-H2O ternary strong electrolyte aqueous solutions and the mass action concentration also strictly follows the mass action law.
S M, CHURNEY K L, NUTTALL R L. The NBS tables of chemical thermodynamic properties-selected values for inorganic and C1 and C2 organic substances in SI units [M]. LIU Tian, ZHAO Meng-yue. Beijing: Chinese Standards Press, 1998. (in Chinese)" target="blank">[19] WAGMAN D D, EVANS W H, EVANS W H, PARKER V B, SCHUMM R H, HALOW I, BAILEY S M, CHURNEY K L, NUTTALL R L. The NBS tables of chemical thermodynamic properties-selected values for inorganic and C1 and C2 organic substances in SI units [M]. LIU Tian, ZHAO Meng-yue. Beijing: Chinese Standards Press, 1998. (in Chinese)


