Trans. Nonferrous Met. Soc. China 26(2016) 581-588
Corrosion and leaching behaviors of Sn-based alloy in simulated soil solutions
Xiao-dong LAO1,2, Cong-qian CHENG1, Xiao-hua MIN1, Jie ZHAO1, Da-yu ZHOU1, Li-hua WANG1, Xiao-gang LI3
1. School of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, China;
2. School of Physics and Electrical Engineering, Zhoukou Normal University, Zhoukou 466000, China;
3. School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, China
Received 8 April 2015; accepted 21 August 2015
Abstract:
The corrosion and leaching behaviors of Sn-0.75Cu solders and joints in NaCl-Na2SO4 and NaCl-Na2SO4-Na2CO3 simulated soil solutions were investigated compared with those in NaCl solution, aiming to assess the potential risk from the electronic-waste disposed in soil. The leaching kinetics of Sn reveals that the leaching amount of Sn increases with increasing the time. The amount of Sn leached from the joint is the largest in NaCl solution.  and
and 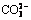 inhibit the leaching of Sn from the joints, but accelerate that from the solders. Meanwhile, the corrosion layer of the joint in NaCl solution is more porous, and those immersed in NaCl-Na2SO4 and NaCl-Na2SO4-Na2CO3 solutions are compact. The XRD results indicate that the main corrosion products on the solders and joints surfaces are comprised of tin oxide, tin chloride and tin chloride hydroxide. The potentiodynamic polarization measurements for the solders were discussed in the simulated soil solutions.
inhibit the leaching of Sn from the joints, but accelerate that from the solders. Meanwhile, the corrosion layer of the joint in NaCl solution is more porous, and those immersed in NaCl-Na2SO4 and NaCl-Na2SO4-Na2CO3 solutions are compact. The XRD results indicate that the main corrosion products on the solders and joints surfaces are comprised of tin oxide, tin chloride and tin chloride hydroxide. The potentiodynamic polarization measurements for the solders were discussed in the simulated soil solutions.
Key words:
Sn-0.75Cu alloy; corrosion; leaching; simulated soil solutions;
1 Introduction
The environmental negative effects of the electronic waste (E-waste) are drawing increasing attention. With the implementation of waste electrical and electronic equipment (WEEE) directive and the restriction of hazardous substances (RoHS) directive [1-3], the lead-free solders have gradually replaced the traditional lead solder to reduce the pollution of heavy metals. The most widely used lead-free solder mainly consists of tin with the addition of other elements such as copper and silver [4].
There are many researches on recovering the waste solders, for example, YANG et al [5] carried out the recycle of waste Sn-based alloys by the industrial experiments of vacuum distillation. However, nowadays most of E-wastes with solders are finally disposed in landfill instead of recovered. The heavy metals of the solder from the E-waste can enter soil and aquatic systems via leaching from dumpsites. Therefore, it can cause severe damage to the environment and threaten human health without any control of E-waste [6-8]. It is essential to assess the environment risk of the waste lead-free solders. However, SnCu has the lowest impact in environment risk assessment than SnAgCu and SnPb according to Ref. [9]. A number of investigations focused on the corrosion behavior of SnCu solder in NaCl solution.  et al [10] discussed the dependence of the scale of microstructure of Sn-0.7Cu solder on the electrochemical corrosion behavior which performed in 3.5% NaCl solution at room temperature. GAO et al [11] reported the corrosion behaviors of Sn-0.75Cu solder and joint in 3.5% NaCl solution by potentiodynamic polarization tests. FARINA and MORANDO [12] found that Sn-0.7Cu solder has better resistance to localized corrosion as well as to general corrosion in 0.6% NaCl solution.
et al [10] discussed the dependence of the scale of microstructure of Sn-0.7Cu solder on the electrochemical corrosion behavior which performed in 3.5% NaCl solution at room temperature. GAO et al [11] reported the corrosion behaviors of Sn-0.75Cu solder and joint in 3.5% NaCl solution by potentiodynamic polarization tests. FARINA and MORANDO [12] found that Sn-0.7Cu solder has better resistance to localized corrosion as well as to general corrosion in 0.6% NaCl solution.
However, there is a lack of studies on the dependence of corrosion characteristic of the solder on the leaching behavior of heavy metals in simulated soil solutions. In our previous researches [4], the corrosion and leaching behaviors of heavy metal elements from SnAgCu, SnZn and SnPb solders have been investigated in typical solutions with different pH values. The results showed that significant amounts of Sn were leached from the solders into the solutions. SMITH and SWANGER [13] also reported the presence of Sn on the leaching toxicity of seven lead-free solders. Sn can make the liver and renal tubular degenerate, and damage the central nervous system [14]. However, further research is also required to investigate the corrosion and leaching behaviors of lead-free solder in the soil. In the present research, the simulated soil solutions were employed, aiming to assess the environment risk from the lead-free solder of E-waste disposed in the soil.
2 Experimental
2.1 Simulated soil solutions
The soluble salt ions of soil, such as Clˉ, 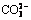 ,
,  and Na+, were considered as the major factors associated with the corrosion performance of soils. Therefore, 2%NaCl-0.6%Na2SO4 and 1%NaCl- 0.6%Na2SO4-3%Na2CO3 mixed solutions were employed to simulate the soil solutions, respectively, and 3.5% NaCl solution was employed for comparison. The 3.5% NaCl solution was prepared by dissolving 10.8 g NaCl directly into 300 mL de-ionized water. The same method was employed to prepare 2%NaCl-0.6%Na2SO4 (NaCl-Na2SO4) and 1%NaCl-0.6%Na2SO4-3%Na2CO3 (NaCl-Na2SO4-Na2CO3) solutions.
and Na+, were considered as the major factors associated with the corrosion performance of soils. Therefore, 2%NaCl-0.6%Na2SO4 and 1%NaCl- 0.6%Na2SO4-3%Na2CO3 mixed solutions were employed to simulate the soil solutions, respectively, and 3.5% NaCl solution was employed for comparison. The 3.5% NaCl solution was prepared by dissolving 10.8 g NaCl directly into 300 mL de-ionized water. The same method was employed to prepare 2%NaCl-0.6%Na2SO4 (NaCl-Na2SO4) and 1%NaCl-0.6%Na2SO4-3%Na2CO3 (NaCl-Na2SO4-Na2CO3) solutions.
2.2 Solder and joint preparation
Sn-0.75Cu solder was prepared by high-purity tin and copper in mass fraction. The mixtures of pure elements were melted at 600 °C in a vacuum furnace. The prepared solders were machined to pieces with 6 mm in diameter and 1.5 mm in thickness. All the pieces were polished with silicon carbide emery paper of grade 400 to simulate the roughness of the actual solder and joint in electronic equipment, and then cleaned with acetone, followed by rinsing with de-ionized water and drying with air drier. Subsequently, the solders were welded with Cu substrate to form the joints by desktop lead-free reflow oven at 250 °C for 60 s.
2.3 Leaching procedure
A schematic diagram of the leaching apparatus is illustrated in Fig. 1. The samples pasted on the end of glass rods were immersed in the reaction tubes with 15 mL simulated soil solutions, respectively. The non-working surface of the specimen was sealed with epoxy resin. All the reaction tubes were placed in the water bath at (45±3) °C to simulate the extremely high temperature of land surface of south area in summer and accelerate the leaching process. Simultaneously, the oxygen was introduced into the test solutions at a rate of 0.77 mL/s for 300 s every 3 d to maintain saturated oxygen concentration. The corresponding fresh solutions were regularly added into the tubes to keep the volume of simulated solutions as 15 mL. The experiments were conducted for 15, 30, 45 and 60 d, respectively.
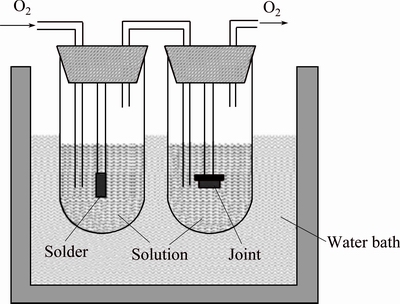
Fig. 1 Schematic diagram of leaching apparatus [4]
After the experiments, the simulated solutions were diluted into a 50 mL volumetric flask with de-ionized water. To avoid Sn dissolving into the solution to form Sn precipitate, the solutions removed from the tubes should be detected immediately. If the precipitate appears in the solutions, sodium phosphate (Na3PO4) solution could be added to dissolve them before analysis, and then the concentration of Sn was detected by atomic absorption spectrophotometer (AAS SOLAN969) with a detection limit at 100 μg/L. The leaching amounts of Sn per unit area of samples were calculated to investigate the leaching kinetics of Sn. The surface morphologies of solders and joints after immersing into different solutions were observed by scanning electron microscopy (SEM JSM5600). The corrosion products were detected by X-ray diffraction (XRD DMAX-2400).
2.4 Electrochemical measurements
The potentiodynamic polarization tests were performed in the simulated soil solutions in a conventional three-electrode cell, using a platinum plate as the counter electrode, a saturated calomel electrode (SCE) as the reference electrode and the solder as the working electrode. All the specimens were polished from 400-grade silicon carbide emery paper up to 1500-grade, rinsed and dried. Before the tests, the oxygen was introduced into the electrolyte for 50 min. The apparatus was placed in the water bath at (45±3) °C to correspond with the leaching procedure. The measurement was conducted with a scan rate of 1 mV/s from -0.8 to 1.8 V after the open-circuit-potential (OCP) reached a relatively steady state.
3 Results and discussion
3.1 Leaching behavior
Figure 2 illustrates the amounts of Sn leached from Sn-0.75Cu solders and their joints after immersion in different simulated solutions for 45 d. The dramatic amounts of Sn leached from the specimens are detected. CHENG et al [4] observed the significant amounts of Sn leached from the solders and joints immersing in H2SO4, NaOH and NaCl solutions. MORI et al [15] reported the dissolution of Sn from the lead-free solders in H2SO4 and HNO3 solutions, respectively. BRENNEN et al [16] found that significant amounts of Sn were leached from the Sn-based solder into tap water and well water. The leaching of Sn from Sn-based solder induced by corrosion should not be neglected due to the potential pollution.
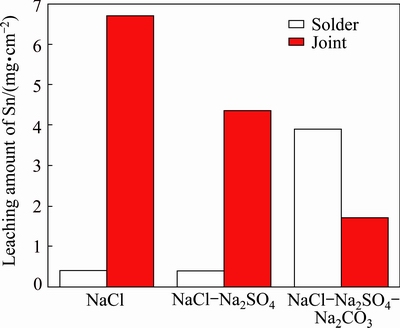
Fig. 2 Leaching amount of Sn from Sn-0.75Cu solders and joints in simulated soil solutions for 45 d
In Fig. 2, the amount of Sn leached from the solder in NaCl solution almost equals that in NaCl-Na2SO4 solution, however, the amount of Sn is the highest in NaCl-Na2SO4-Na2CO3 solution. On the contrary, the amount of Sn leached from the joint in NaCl is the highest, but moderate amount is present in NaCl-Na2SO4 solution and less amount in NaCl-Na2SO4-Na2CO3 solution. The amounts of Sn from the solders in NaCl and NaCl-Na2SO4 solution are significantly lower than those from the joints; by contrast, the amount of Sn leached from the solder is higher than that leached from the joint in NaCl-Na2SO4-Na2CO3 solution.
The leaching performance of solders and joints is influenced apparently by the presence of Clˉ, 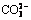 and
and  ions. These results reveal that the addition of Clˉ ions promotes the leaching amounts of Sn from the joints, whereas the additions of
ions. These results reveal that the addition of Clˉ ions promotes the leaching amounts of Sn from the joints, whereas the additions of  and
and 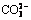 ions into NaCl solution inhibit the dissolution of Sn, but the performance of the solders is quite opposite to that of the joints.
ions into NaCl solution inhibit the dissolution of Sn, but the performance of the solders is quite opposite to that of the joints.
3.2 Leaching kinetics
Figure 3 illustrates the correlation between the leaching amounts of Sn from Sn-0.75Cu solders and joints and exposure time in three solutions.
The leaching amounts of Sn from the solder in NaCl solution increase slowly with increasing the exposure time, and a similar leaching characteristic is observed for the solder in NaCl-Na2SO4 solution. Meanwhile, the amounts of Sn leached from the solder in NaCl- Na2SO4-Na2CO3 solution increase gradually during the exposure time, and are significantly higher than those in other two solutions.
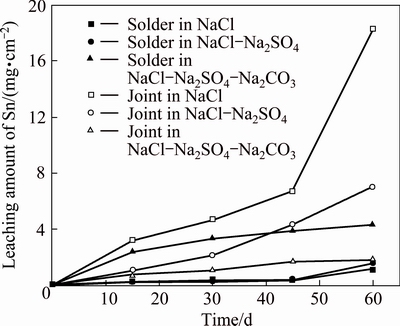
Fig. 3 Leaching kinetics of Sn from Sn-0.75Cu solders and joints in simulated soil solutions
The amount of Sn leached from the joint in NaCl solution increases significantly with increasing the immersion time, and is the highest among those leached from the solder and joint in different solutions. The amount of Sn released from the joint in NaCl-Na2SO4 solution increases steadily, while the amount of Sn increases slowly in NaCl-Na2SO4-Na2CO3 solution.
A similar leaching characteristic is observed for the solders compared with the joints. The leaching amounts of Sn increase obviously with increasing the exposure time for the solders and joints. The results indicate that the presence of  and
and 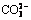 inhibits the dissolution of Sn from the joints, but facilitates that from the solders.
inhibits the dissolution of Sn from the joints, but facilitates that from the solders.
3.3 Surface morphology
The surfaces of solders and joints immersed in different simulated soil solutions for 45 d were observed by SEM. The typical images clearly reveal that the surfaces of solders and joints are covered by a layer of corrosion products, as shown in Fig. 4.
In NaCl solution, the surface of solder is covered apparently by a corrosion layer with small cracks in Fig. 4(a). Meanwhile, the surface of the joint is not compact and appears some cracks, indicating that the corrosion product can be peeled off from the substrate, as shown in Fig. 4(b). The corrosion layer formed on the solder surface shows a shielding effect on the Sn ion transport of the solder and solution. By contrast, the loose layer on the joint surface shows the opposite result, in this case, the corrosion products would no longer protect the substrate [17], resulting in plenty of leaching amount of Sn from the solder. The results correspond well to the leaching amounts of Sn from solders and joints, as presented in Fig. 2.
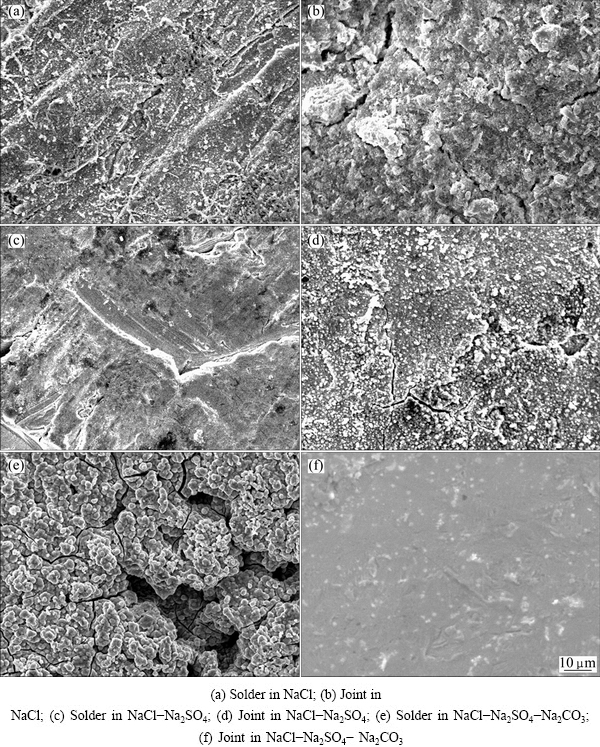
Fig. 4 Microstructures of Sn-0.75Cu solders and joints immersed in simulated soil solutions for 45 d
The surface images of the solder and joint after immersion in NaCl-Na2SO4 solution are illustrated in Figs. 4(c) and (d), respectively. The surface films are not uniform in thickness although both the solder and joint are covered by a corrosion layer. However, the surface of joint is much more micro-cracked, indicating that the corrosion layer presents less protective properties compared with that on the solder [18], it is the reason that the leaching amount of Sn from the joint is higher than that from the solder, as shown in Fig. 2.
In Figs. 4(e) and (f), the surfaces of solder and joint are observed after immersion in NaCl-Na2SO4-Na2CO3 solution. The surface of solder appears with larger granules and many apparent cracks which are attributed to the leaching of Sn from the solder. However, the surface of joint is so uniform and compact that the leaching amount of Sn reduces, as shown in Fig. 2.
The cross-section images of corrosion layers for the solder and joint after tested in NaCl-Na2SO4-Na2CO3 solution for 45 d are illustrated in Fig. 5. It clearly reveals that the solder surface is well covered by a layer of corrosion products with a thickness of 20-40 μm, as shown in Fig. 5(a). Apparently, the loose corrosion layer appears many cracks extending from the surface of the corrosion film towards the film/solder interface, resulting in the dramatic leaching amount of Sn from the solder. As shown in Fig. 5(b), the corrosion layer of the joint is thinner than that of the solder, but is more compact. It inhibits the dissolution of Sn from the joint.
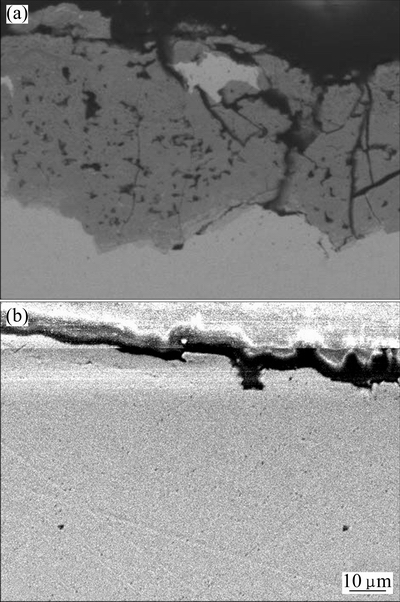
Fig. 5 Cross-section images of corrosion products of Sn-0.75Cu solder (a) and joint (b) after leaching tests in NaCl-Na2SO4-Na2CO3 solution for 45 d
3.4 Surface corrosion products
Figure 6 and Table 1 show the XRD results of corrosion products of the solders after immersion in three solutions for 45 d. In NaCl solution, the corresponding peaks of tin oxide (SnO) and tin chloride (SnCl4) are detected in the corrosion layer. Meanwhile, the existence of tin oxide (SnO) is observed in NaCl-Na2SO4 solution due to the thin corrosion layer. The corrosion layer in NaCl-Na2SO4-Na2CO3 solution is composed of tin oxide (SnO), tin chloride (SnCl4) and tin chloride hydroxide (Sn4(OH)6Cl2). The presence of tin chloride hydroxide as corrosion product of lead-free solder has been reported by CHENG et al [4] and ROSALBINO et al [19].
The XRD results obtained from the joints are also shown in Fig. 7 for comparison. The corrosion products on the joint in NaCl solution expose the peaks of tin chloride (SnCl2) and tin chloride hydroxide (Sn4(OH)6Cl2). The corrosion products are comprised of tin oxide (SnO2) and tin chloride hydroxide (Sn4(OH)6Cl2) in NaCl-Na2SO4 solution, while the peaks of substrate are not strong because of much thicker corrosion layer. The corrosion product in NaCl-Na2SO4- Na2CO3 solution is not shown in Fig. 7, because the corrosion layer is so thin that the corresponding peaks are not observed except the substrate.

Fig. 6 XRD patterns of surface products on Sn-0.75Cu solders immersed in simulated soil solutions for 45 d
Table 1 Surface corrosion products on Sn-0.75Cu solders and joints immersed in simulated soil solutions for 45 d
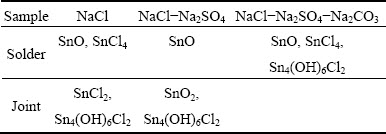
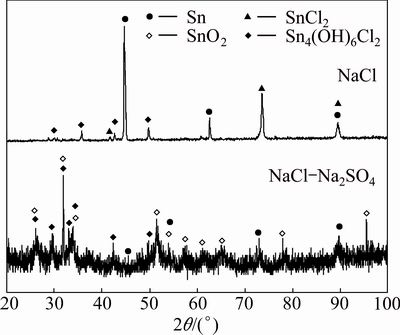
Fig. 7 XRD patterns of surface products on Sn-0.75Cu joints immersed in simulated soil solutions for 45 d
3.5 Discussion
3.5.1 Effect of solution components on solder
The potentiodynamic polarization curves of Sn-0.75Cu solder alloys in the simulated soil solutions are shown in Fig. 8. The corrosion current density (Jcorr) and potential (φcorr) are obtained using both the cathodic and anodic branches of Tafel’s extrapolation, as summarized in Table 2. The polarization curves with similar shapes for the solders in NaCl and NaCl-Na2SO4 solutions are observed. The corrosion current density of solder in NaCl solution is nearly the same as that in NaCl-Na2SO4 solution. The corrosion current density of solder in NaCl-Na2SO4-Na2CO3 solution shows a significant shift towards more positive than those in NaCl and NaCl-Na2SO4 solutions. The solders immersed in NaCl and NaCl-Na2SO4 solutions exhibit better corrosion resistance than that in NaCl-Na2SO4-Na2CO3 solution, due to lower corrosion current density and more stable corrosion films on the surfaces [17]. This seems to explain why the leaching amount of Sn from the solders in NaCl solution is similar with that in NaCl-Na2SO4 solution, and lower than that in NaCl-Na2SO4-Na2CO3 solution (Figs. 2 and 3).
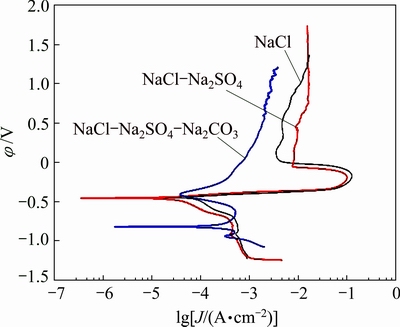
Fig. 8 Potentiodynamic polarization curves of Sn-0.75Cu solder alloys in simulated soil solutions
Table 2 Electrochemical properties of solder alloys in simulated soil solutions after polarization test

The cathode regions of polarization curves correspond with the reduction reaction of dissolved oxygen to produce hydroxyl ions [20], which is expressed as follows:
O2+2H2O+4e=4OH- (1)
The active dissolution of solder continues with increasing the potential until the current density reaches a critical value (0.1 A/cm2) in anode region in NaCl and NaCl-Na2SO4 solutions. Several studies on the corrosion behavior of lead-free solder have been reported in Refs. [4,21-23] to explain the dissolution process of Sn which is easily dissolved to form Sn2+ and Sn4+ by the following processes:
Sn=Sn2++2e (2)
Sn2+=Sn4++2e (3)
Then, the passivation starts at the critical point where the current density decreases with increasing the potential [24]. There is a passive process for the solders in NaCl and NaCl-Na2SO4 solutions beginning at 7 mV and -51 mV, respectively.
SnCl2 is observed on the surface of joint in NaCl solution, and SnO2 is observed in NaCl-Na2SO4 solution as listed in Table 1. However, tin chloride hydroxide (Sn4(OH)6Cl2) is also detected on the surface of joints in NaCl and NaCl-Na2SO4 solutions. In this case, the formation of tin chloride hydroxide is given by the following process [25,26]:
4Sn+2Cl-+6OH--8e=Sn4(OH)6Cl2 (4)
The polarization of solder in NaCl-Na2SO4- Na2CO3 solution is different with those in NaCl and NaCl-Na2SO4 solutions. The current density increases until 0.5 mA/cm2 in active dissolution region in NaCl- Na2SO4-Na2CO3 solution with increasing the potential from -800 to -630 mV. Beyond this point, the current density decreases, and the formation of tin compounds is contributed to this decrease corresponding to the passivation region. Then, the sharp increase of current density may be attributed to the breakdown of passivation film.
3.5.2 Effect of solution components on joint
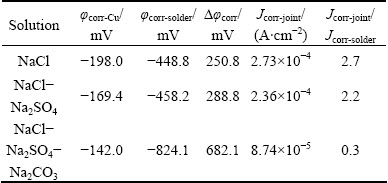
The potentiodynamic polarization curves of Cu are compared with those of solders in the simulated soil solutions, as shown in Fig. 9. The electrochemical properties are summarized in Table 3. The current density of joint in NaCl solution is similar to that in NaCl-Na2SO4 solution (Figs. 9 (a) and (b)). The current density of the joint is almost two folds higher than that of the solder (Table 3). The cathodic branch of Cu intersects the active region of the anodic branch of solder. The galvanic cell accelerates the leaching amount of Sn from the joint, due to higher galvanic corrosion current density of joint compared with that of solder [27]. The oxidation potentials of Cu/Cu2+ and Sn/Sn2+ are 0.345 V (vs SHE) and -0.136 V (vs SHE), respectively [28]. As a result, Sn acting as an anode with lower corrosion potential will be corroded preferentially in the joint which is comprised of solder and copper as the galvanic cell. Consequently, the corrosion process of joint can be accelerated. Copper as the other solder constituent does not affect the corrosion reaction significantly according to the mass of Cu in the solder and the experimental results [15,28].
However, the cathodic branch of Cu intersects the passive region of solder in NaCl-Na2SO4-Na2CO3 solution (Fig. 9 (c)), where the galvanic corrosion current density of joint is 3/10 of that of solder (Table 3), indicating that the corrosion reaction is inhibited, and consequently the growth of corrosion product is slowed down [28], thus the dissolution of Sn is greatly suppressed, as shown in Fig. 2.
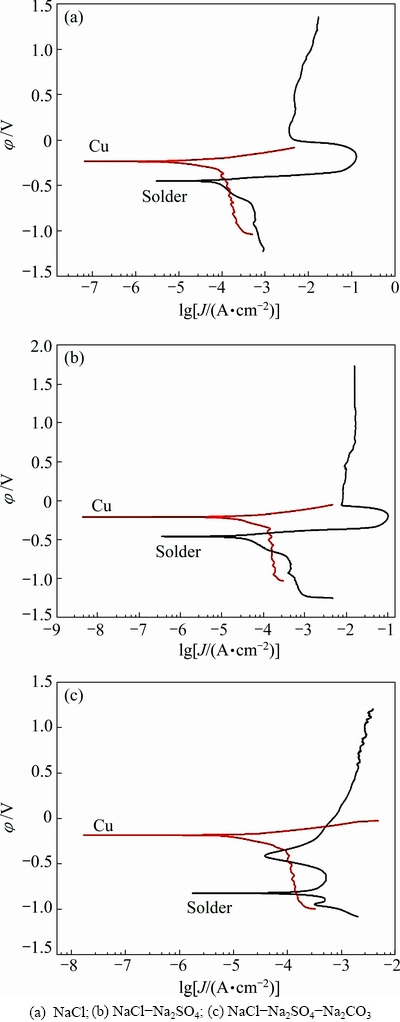
Fig. 9 Potentiodynamic polarization curves of Sn-0.75Cu solder alloys and Cu in simulated soil solutions
Table 3 Electrochemical properties of joints in simulated soil solutions after polarization test
Meanwhile, the solution components attribute to the corrosion behavior of joints. The leaching amounts of Sn from joints are generally higher than those from solders except in NaCl-Na2SO4-Na2CO3 solution. On the surfaces of joints in NaCl and NaCl-Na2SO4 solutions, the corrosion product layers are so loose and porous that the layers do not play a role in protection (Figs. 4(b) and (d)), leading to the acceleration of the leaching of Sn. However, insignificant amounts of Sn are leached from the joints in NaCl-Na2SO4-Na2CO3 solution, because the corrosion layer formed on the surface is more stable and compact (Fig. 4(f)) than that in other solutions. In addition, the corrosion layer of joint in NaCl-Na2SO4- Na2CO3 solution acts as a barrier layer to inhibit further leaching of Sn during the test period [18].
4 Conclusions
1) The leaching amount of Sn gradually increases with increasing the exposure time in the simulated soil solutions. Under the influence of NaCl and NaCl-Na2SO4 solutions, the corrosion product layers of the joints are so loose and porous that they do not play a role in protection, and then accelerate the leaching of Sn. The presence of NaCl-Na2SO4-Na2CO3 solution contributes to the compact corrosion layer of the joint, resulting in the decrease of leaching amount of Sn.
2) The major corrosion products are comprised of tin oxide, tin chloride and tin chloride hydroxide on the surface after immersion in the test solutions for 45 d.
3) The potentiodynamic polarization curves of solder and Cu correspond with the leaching behaviors of Sn from the solders and joints.
References
[1] YANG Jian-xin, LU Bin, XU Cheng. WEEE flow and mitigating measures in China [J]. Waste Management, 2008, 28(9): 1589-1597.
[2] EVELOY V, GANESAN S, FUKUDA Y, WU J, PECHT M G. WEEE, RoHS, and what you must do to get ready for lead-free electronics [C]//Proceedings of the Sixth International Conference on Electronics Packaging Technology. Piscataway: IEEE, 2005: 27-44.
[3] EVELOY V, GANESAN S, FUKUDA Y, WU J, PECHT M G. Are you ready for lead-free electronics? [J]. IEEE Transactions on Components and Packaging Technologies, 2005, 28(4): 884-894.
[4] CHENG C Q, YANG F, ZHAO J, WANG L H, LI X G. Leaching of heavy metal elements in solder alloys [J]. Corrosion Science, 2011, 53(5): 1738-1747.
[5] YANG Bin, KONG Ling-xin, XU Bao-qiang, LIU Da-chun, DAI Yong-nian. Recycling of metals from waste Sn-based alloys by vacuum separation [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1315-1324.
[6] WIDMER R, OSWALD-KRAPF H, SINHA-KHETRIWAL D, SCHNELLMANN M,  H. Global perspectives on e-waste [J]. Environmental Impact Assessment Review, 2005, 25(5): 436-458.
H. Global perspectives on e-waste [J]. Environmental Impact Assessment Review, 2005, 25(5): 436-458.
[7] BABU B R, PARANDE A K, BASHA C A. Electrical and electronic waste: A global environmental problem [J]. Waste Management & Research, 2007, 25(4): 307-318.
[8] ROBINSON B H. E-waste: An assessment of global production and environmental impacts [J]. Science of the Total Environment, 2009, 408(2): 183-191.
[9] SOCOLOF M L, GEIBIG J R. Life-cycle impacts of lead and lead-free solder used in wave soldering of electronics [C]//Proceedings of the 2005 IEEE International Symposium on Electronics and the Environment. Piscataway: IEEE, 2005: 96-101.
[10]  W R, FREITAS E S, SPINELLI J E, GARCIA A. Electrochemical behavior of a lead-free Sn-Cu solder alloy in NaCl solution [J]. Corrosion Science, 2014, 80: 71-81.
W R, FREITAS E S, SPINELLI J E, GARCIA A. Electrochemical behavior of a lead-free Sn-Cu solder alloy in NaCl solution [J]. Corrosion Science, 2014, 80: 71-81.
[11] GAO Yan-fang, CHENG Cong-qian, ZHAO Jie, WANG Li-hua, LI Xiao-gang. Electrochemical corrosion of Sn-0.75Cu solder joints in NaCl solution [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(4): 977-982.
[12] FARINA S, MORANDO C. Comparative corrosion behaviour of different Sn-based solder alloys [J]. Journal of Materials Science: Materials in Electronics, 2015, 26(1): 464-471.
[13] SMITH E B, SWANGER L K. Toxicity and worldwide environmental regulation of lead-free solders [J]. Transactions of the Institute of Metal Finishing, 2000, 78(2): B18-B21.
[14] HE Bin, YUN Zhao-jun, SHI Jian-bo, JIANG Gui-bin. Research progress of heavy metal pollution in China: Sources, analytical methods, status, and toxicity [J]. Chinese Science Bulletin, 2013, 58(2): 134-140.
[15] MORI M, MIURA K, SASAKI T, OHTSUKA T. Corrosion of tin alloys in sulfuric and nitric acids [J]. Corrosion Science, 2002, 44(4): 887-898.
[16] BRENNEN M, PERUMAREDDI J R, SASTRI V S, ELBOUJDAINI M, BROWN J R. Studies on leaching of metals from solders due to corrosion [J]. Materials and Corrosion, 1998, 49(8): 551-555.
[17] LI D Z, CONWAY P P, LIU C Q. Corrosion characterization of tin-lead and lead free solders in 3.5 wt.% NaCl solution [J]. Corrosion Science, 2008, 50(4): 995-1004.
[18] LIU J, PARK S, NAGAO S, NOGI M, KOGA H, MA J, ZHANG G, SUGANUMA K. The role of Zn precipitates and Cl- anions in pitting corrosion of Sn-Zn solder alloys [J]. Corrosion Science, 2015, 92: 263-271.
[19] ROSALBINO F, ANGELINI E, ZANICCHI G, MARAZZA R. Corrosion behaviour assessment of lead-free Sn-Ag-M (M=In, Bi, Cu) solder alloys [J]. Materials Chemistry and Physics, 2008, 109(2-3): 386-391.
[20] NAZERI M F M, ISMAIL A B, MOHAMAD A A. Effect of polarizations on Sn-Zn solders alloys in alkaline electrolyte [J]. Journal of Alloys and Compounds, 2014, 606: 278-287.
[21] MOHANTY U S, LIN K. The effect of alloying element gallium on the polarization characteristics of Pb-free Sn-Zn-Ag-Al-XGa solders in NaCl solution [J]. Corrosion Science, 2006, 48(3): 662-678.
[22] JUNG J, LEE S, JOO Y, LEE H, PARK Y. Anodic dissolution characteristics and electrochemical migration lifetimes of Sn solder in NaCl and Na2SO4 solutions [J]. Microelectronic Engineering, 2008, 85(7): 1597-1602.
[23] WANG M N, WANG J Q, KE W. Corrosion behavior of Sn-3.0Ag-0.5Cu solder under high-temperature and high-humidity condition [J]. Journal of Materials Science: Materials in Electronics, 2014, 25(3): 1228-1236.
[24] MOUANGA M, BERCOT P. Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions. Part II: Electrochemical analyses [J]. Corrosion Science, 2010, 52(12): 3993-4000.
[25] WANG L H, CHENG C Q, YANG F, ZHAO J. Leaching behavior of lead-free solders in a NaCl-Na2SO4-Na2CO3 mixed solution as simulated soil [J]. Journal of Chinese Society for Corrosion and Protection, 2011, 31(5): 381-384. (in Chinese)
[26] MOUANGA M,  P, RAUCH J Y. Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions. Part I: Corrosion layer characterization [J]. Corrosion Science, 2010, 52(12): 3984-3992.
P, RAUCH J Y. Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions. Part I: Corrosion layer characterization [J]. Corrosion Science, 2010, 52(12): 3984-3992.
[27] WANG Bao-cheng. Corrosion and protection of materials [M]. Beijing: Peking University Press, 2012: 118-123. (in Chinese)
[28] CHANG H, CHEN H, LI M, WANG L, FU Y. Generation of tin(II) oxide crystals on lead-free solder joints in deionized water [J]. Journal of Electronic Materials, 2009, 38(10): 2170-2178.
锡基合金在模拟土壤溶液中的腐蚀与浸出
劳晓东1,2,程从前1,闵小华1,赵 杰1,周大雨1,王丽华1,李晓刚3
1. 大连理工大学 材料科学与工程学院,大连 116024;
2. 周口师范学院 物理与机电工程学院,周口 466000;
3. 北京科技大学 材料科学与工程学院,北京 100083
摘 要:研究Sn-0.75Cu焊料合金及其接头在NaCl-Na2SO4和NaCl-Na2SO4-Na2CO3模拟土壤溶液中的腐蚀与浸出行为,并与其在NaCl溶液中的行为进行对比,以评估废弃电子产品被填埋时对环境的潜在危害。Sn的浸出动力学表明,Sn的浸出量随时间的增加而增加。在NaCl溶液中接头的Sn浸出量是最多的。 和
和 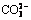 阻碍了接头中Sn的浸出,但加速了焊料中Sn的浸出。在NaCl溶液中的接头表面腐蚀层疏松多孔,而在NaCl-Na2SO4和NaCl-Na2SO4-Na2CO3溶液中的接头表面腐蚀层则较为致密。XRD结果表明,焊料和接头表面的腐蚀产物以Sn的氧化物、氯化物和碱式氯化物为主。讨论了焊料合金在模拟土壤溶液中的动电位极化曲线。
阻碍了接头中Sn的浸出,但加速了焊料中Sn的浸出。在NaCl溶液中的接头表面腐蚀层疏松多孔,而在NaCl-Na2SO4和NaCl-Na2SO4-Na2CO3溶液中的接头表面腐蚀层则较为致密。XRD结果表明,焊料和接头表面的腐蚀产物以Sn的氧化物、氯化物和碱式氯化物为主。讨论了焊料合金在模拟土壤溶液中的动电位极化曲线。
关键词:Sn-0.75Cu合金;腐蚀;浸出;模拟土壤溶液
(Edited by Mu-lan QIN)
Foundation item: Project (2012FY113000) supported by the National Science and Technology Basic Project of the Ministry of Science and Technology of China; Projects (51171037, 51134013, 51101024) supported by the National Natural Science Foundation of China; Project (14B430009) supported by the Science Research Fund of Education Department of Henan Province, China
Corresponding author: Jie ZHAO; Tel: +86-411-84709076; Fax: +86-411-84709284; E-mail: jiezhao@dlut.edu.cn
DOI: 10.1016/S1003-6326(16)64146-8
Abstract: The corrosion and leaching behaviors of Sn-0.75Cu solders and joints in NaCl-Na2SO4 and NaCl-Na2SO4-Na2CO3 simulated soil solutions were investigated compared with those in NaCl solution, aiming to assess the potential risk from the electronic-waste disposed in soil. The leaching kinetics of Sn reveals that the leaching amount of Sn increases with increasing the time. The amount of Sn leached from the joint is the largest in NaCl solution.  and
and 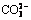 inhibit the leaching of Sn from the joints, but accelerate that from the solders. Meanwhile, the corrosion layer of the joint in NaCl solution is more porous, and those immersed in NaCl-Na2SO4 and NaCl-Na2SO4-Na2CO3 solutions are compact. The XRD results indicate that the main corrosion products on the solders and joints surfaces are comprised of tin oxide, tin chloride and tin chloride hydroxide. The potentiodynamic polarization measurements for the solders were discussed in the simulated soil solutions.
inhibit the leaching of Sn from the joints, but accelerate that from the solders. Meanwhile, the corrosion layer of the joint in NaCl solution is more porous, and those immersed in NaCl-Na2SO4 and NaCl-Na2SO4-Na2CO3 solutions are compact. The XRD results indicate that the main corrosion products on the solders and joints surfaces are comprised of tin oxide, tin chloride and tin chloride hydroxide. The potentiodynamic polarization measurements for the solders were discussed in the simulated soil solutions.


