Trans. Nonferrous Met. Soc. China 24(2014) 3987-3993
Thermodynamics for chemical vapor synthesis of Nb nanopowder in NbCl5-H2-Ar system
Jun ZHU, Kai HUANG, Jun-gang HOU, Hong-min ZHU
Beijing Key Laboratory of Green Recycling and Extraction of Metals, School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China
Received 10 February 2014; accepted 15 August 2014
Abstract:
Thermodynamics for chemical vapor synthesis (CVS) of Nb nanopowder in NbCl5-H2-Ar system was investigated by using FactSage software. The validation experiments were conducted to confirm the thermodynamics points. The results indicate that under the atmospheric pressure, the reduction approach from NbCl5(g) to Nb(s) is a stage-wise process with the formation of complex sub-chlorides, and is controllable at low hydrogen ratio (mole ratio of n(NbCl5):n(H2)<1:180) and low temperature (<1050 °C). Furthermore, a reasonable amount of inert loading gas is favorable to increase the reduction ratio of NbCl5 and the powder yield. The as-synthesized Nb nanopowder with the homogeneous size of 30-50 nm and the powder yield of 85% (mass fraction) is obtained by the CVS process under n(NbCl5):n(H2):n(Ar)=1:120:1 and 950 °C with the NbCl5 reduction rate of 96.1%.
Key words:
NbCl5-H2-Ar system; chemical vapor synthesis (CVS); thermodynamic; niobium nanopowder; FactSage software;
1 Introduction
Niobium is a V sub-group element in periodic table with similarity to tantalum in physical and chemical properties. The largest application of the two metals is as raw materials for high performance solid-electrolyte capacitor with miniaturization and high capacitance in electronic appliances [1,2]. In order to meet the considerable development during these years, Nb or Ta powders with high CV (capacitance times voltage constant) are demanded, and correspondingly various approaches have been developed, such as purifying the powder [3,4], modifying the particle surface [3,5], doping with nitrogen to stabilize the dielectric oxide film [6,7], and controlling size distribution and morphology of the particles [4].
Capacitance value (C) of a capacitor is determined by anode surface area (A) and dielectric thickness (d) (oxide film of anodic particles) [8]:
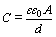 (1)
(1)
where ε is dielectric constant of oxide, and ε0 is dielectric constant of free space. Anode of a capacitor is usually prepared by sintering of Nb or Ta powder with powder metallurgy process. Assuming that the anode is constructed by tangent spheric particles with uniform size and density, as shown in Fig. 1, the anode surface area can be estimated approximately by
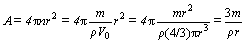 (2)
(2)
where m is anode mass of the capacitor; ρ is density of a single particle; r is radius of a single particle; V0 is volume of a single particle; n is particles number. From Eqs. (1) and (2), it can be concluded that for finer particles, a capacitor with the same capacitance can be manufactured smaller due to the decreasing m, or a higher CV value can be approached with the same anode mass due to the increasing A. That is, preparation of finer powder (e.g., nanopowder) is another critical way to design new capacitors with higher volumetric efficiency. In the market, the newest Ta capacitor shows capacitance exceeding 0.2 FV/g with BET surface area of anode >5.0 m2/g (equivalently to the average size of anode powder <70 nm) [9]. Moreover, nanopowder with larger surface area can improve the sinterability of the powder [10]. Nb or Ta nanopowder can be synthesized by method of metallothermic reduction, such as sodium reduction of K2NbF7/K2TaF7 (Hunter process) [1,4], alumino-, magnesio-, or calcio-thermic reduction of pentoxides [11,12]. However, it is difficult to control size and morphology of the powder in these processes, because strong exothermal reactions will result in agglomeration and uncontrollable growth of the primary grains [13]. The heat effect of the reactions can be mitigated by electrolysis [14] or electronically mediated reaction (EMR) [15] in molten salt, but separation and collection of the powder from molten salt is a crucial challenge. Homogeneous reaction technology in liquid or gas is a hopeful approach to improve the above mentioned problems, and Ta powders with the homogeneous size of 20-60 nm have been prepared by sodium reduction of TaCl5 in liquid ammonia [16,17]. Chemical vapor synthesis (CVS), i.e., deposition (CVD) or condensation (CVC), is another homogeneous reaction process in gas. Nanosized Nb or Ta products by CVS process in MCl5-H2 system have been reported [18-20], but they are seriously suffering from the disadvantages of ultrahigh hydrogen ratio (mole ratio of hydrogen to pentachloride more than 300:1), high reduction temperature (>1400 °C for reduction ratio of the pentachloride no less than 98%, mole fraction), and unknown powder yield (no report in the literature). All the disadvantages should be ascribed to the lack of detailed understanding the thermodynamic and reaction stages of the hydrogen reduction process.
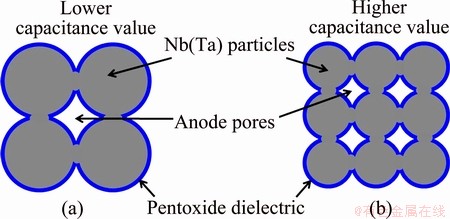
Fig. 1 Schematic of two anodes from different sizes of powder with different capacitance values
Equilibrium thermodynamic calculation based on the total Gibbs free energy minimization is a significant tool to understand the details of a chemical reaction system with complex intermediates [21]. It can be used to evaluate the possibility of the complicated gas-phase chemical reactions [22], to indicate the formation mechanism of the condensed phases under different operating conditions [23,24], and to predict the optimal synthesis conditions during the process of hard materials prepared in M-H-Cl(F) system [25]. The authors have calculated the equilibrium phase states of NbCl5-H2-Ar system in different operating conditions by using SimuSage software [26], but the details of the reduction process were not completely understood.
In this work, a complete thermodynamic equilibrium analysis of NbCl5-H2-Ar system was performed in the conditions given by Equilib module in FactSage software. This software is designed based on the Gibbs free energy minimization [27]. The effects of the synthesis parameters (e.g., reduction temperature, hydrogen mole ratio and loading gas amount) on the equilibrium phases, composition and content were fully studied, and the stage of the hydrogen reduction was discussed according to the thermodynamics. Finally, the relative experiments were conducted in order to validate the thermodynamics.
2 Thermodynamic calculation
The minimization free energy (G) of a complex CVS system can be expressed as [21]
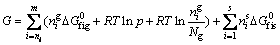 (3)
(3)
where m is the number of gaseous species; s is the number of solid phases; nig is the mole amount of gaseous species; nis is the mole amount of solid species; Ng is the total mole amount of gaseous species; p is the total pressure; ΔG0fig is the formation free energy of a gaseous species; ΔG0fis is the formation free energy of a solid species.
In Equilib program of FactSage, NbCl5(g), H2(g) and Ar(g) were selected as reactant precursors, where Ar(g) was an inert gas to load the vapor of the pentachloride. The setting reaction conditions were: temperature 200-3000 °C, hydrogen ratio (mole ratio of hydrogen to pentachloride) (2.5-200):1, and mole ratio of Ar to pentachloride (0-100):1, as well as the amount of NbCl5(g) fixed at 1 mol and the total pressure set at isobaric 101325 Pa. The gaseous and condensed phases selected for the calculation are listed in Table 1, and thermodynamic data of these species have already been included in the internal database of the software. Then, the equilibrium content of these species as a function of the temperature and the hydrogen ratio can be obtained.
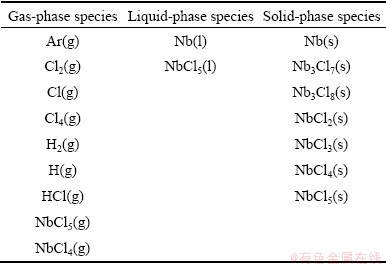
Table 1 Species selected in thermodynamic calculations of NbCl5-H2-Ar system
3 Results and discussion
3.1 Composition diagram of equilibrium phase in NbCl5-H2 binary system
Equilibrium phases were firstly ascertained in NbCl5-H2 binary system. The composition diagrams of the equilibrium phases were constructed by plotting the amount of the species as a function of the temperature, as shown in Fig. 2 and Fig. 3. Only the equilibrium phases with content more than 10-4 mol were shown.
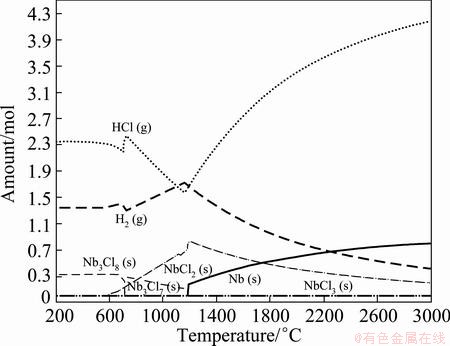
Fig. 2 Equilibrium phases distribution of species in NbCl5-H2 binary system with n(H2):n(NbCl5)=2.5:1 at 101325 Pa
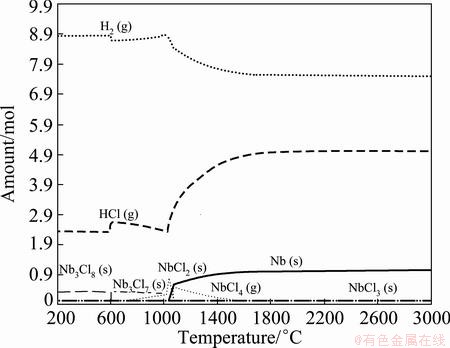
Fig. 3 Equilibrium phases distribution of species in NbCl5-H2 binary system with n(H2):n(NbCl5)=10:1 at 101325 Pa
In Fig. 2, when the temperature is in the range of 200-1180 °C, the main products are NbCl4(g), Nb3Cl7(s), Nb3Cl8(s) or NbCl2(s) in the binary system with n(H2):n(NbCl5)=2.5:1 at 101325 Pa. The Nb phase is formed until the temperature rising up to 1180 °C, and its content increases with the increase of the temperature. When the temperature is up to 3000 °C, the amount of Nb in the equilibrium phase is 0.8 mol, equivalently to the Nb yield (ηNb) of 80%. Here,
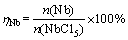 (4)
(4)
where n(Nb) is the amount of Nb in equilibrium phases, n(NaCl5) is the amount of NbCl5 precursor.
This means that it is very difficult to produce purified Nb metal just by increasing temperature. However, with the increase of the hydrogen ratio from 2.5 to 10 (see Fig. 3), the formation temperature of Nb decreases from 1180 °C to 1038 °C, and even decreases from 3000 °C to 1460 °C for the achievement of ηNb of 90%. This indicates that a higher hydrogen ratio can facilitate the formation of Nb with more yields at lower temperature.
Furthermore, as shown in Fig. 2 and Fig. 3, the reaction stages could be proposed as follows:
Firstly, at 200-600 °C, a primary reaction happens,
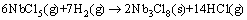 (5)
(5)
The generated Nb3Cl8 could be consumed partially at about 600 °C by
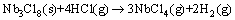 (6)
(6)
and consumed mostly at 700 °C by
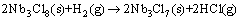 (7)
(7)
Then from 700 °C to 1160 °C, the reaction should be
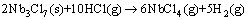 (8)
(8)
At 1160-1180 °C, a new reaction would start to consume the remanent Nb3Cl7,
 (9)
(9)
The NbCl2 would decompose at 1180-1200 °C,
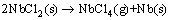 (10)
(10)
Finally, Nb metal could be generated by the reaction of NbCl4 with hydrogen,
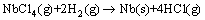 (11)
(11)
According to the reactions (10) and (11), it should be responsible that the direct precursor for the formation of Nb(s) is NbCl4(g) or NbCl2(s) instead of NbCl5(g). Moreover, the variation of hydrogen ratio should have no effect on the stages except for influencing the temperature of the reactions.
3.2 Effects of hydrogen ratio and temperature
According to the results of Fig. 2 and Fig. 3, it suggests that higher hydrogen ratio should be benefit to the formation of Nb. However, it is necessary to ascertain whether an ultrahigh hydrogen ratio should be applied. For this purpose, the effects of hydrogen ratio and temperature on ηNb were investigated at equilibrium conditions, and the results are shown in Fig. 4 and Fig. 5. As can be seen, higher hydrogen ratio increases the value of ηNb and decreases the reduction temperature. For example, the value of ηNb approaches to 98% at 1050 °C with hydrogen ratio of 60:1. However, the temperature needs up to 1500 °C at the same value of ηNb with hydrogen ratio of 10:1, otherwise, the value of ηNb is less than 20% at 1050 °C. But it is hard to detect the abovementioned influence when the hydrogen ratio exceeds 80:1 and the temperature is up to 1100 °C (see Fig. 5), which means that continuously increasing both of the hydrogen ratio and the temperature should not be efficient anymore.
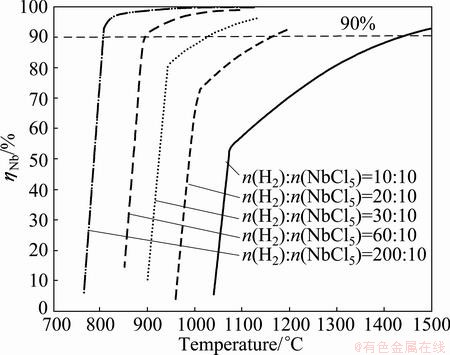
Fig. 4 Yield of Nb (ηNb) as function of temperature in equilibrium NbCl5-H2 system at different hydrogen ratios
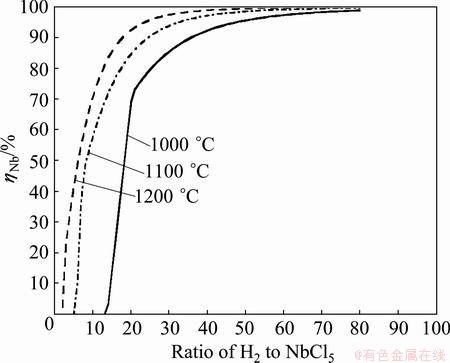
Fig. 5 Effect of hydrogen ratio on yield of Nb (ηNb) at different temperatures in equilibrium NbCl5-H2 system
From the results of the thermodynamics, NbCl5 can be reduced to Nb with the yield of Nb more than 99.9% in the conditions of hydrogen ratio <180:1 and temperature <1050 °C. It is necessary to pursue neither large hydrogen ratio nor high temperature.
3.3 Effect of loading gas argon
The influence of content of Ar on ηNb in NbCl5-H2-Ar equilibrium system was evaluated, as shown in Fig. 6. At lower hydrogen ratio (see Fig. 6(a)) and lower temperature, the increase of the content of Ar is benefit to improve the value of ηNb. The reason should be ascribed to the decrease of partial pressure of the equilibrium system caused by the inert gas addition.

Fig. 6 Effect of content of Ar on yield of Nb (ηNb) with n(H2):n(NbCl5)=10:1 (a) and 30:1 (b) at different temperatures in equilibrium NbCl5-H2-Ar system
The overall chemical reaction of the hydrogen reduction process of NbCl5 can be expressed as
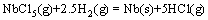 (12)
(12)
The role of argon in this work is inert gas to load the vaporization of the pentachloride into the reactor. The stoichiometric coefficient of the gaseous species in reaction (12) is (5-2.5-1)=1.5>0. This means that the partial pressure of the products is larger than that of the reactants when the reaction is in equilibrium. When inert gas is added into the NbCl5-H2 binary system at constant temperature and constant pressure, the partial pressure of the equilibrium system decreases. According to the principle of chemical equilibrium, the equilibrium of the reaction moves towards the direction of increasing the partial pressure to counteract the influence of the inert gas addition. That is, the yield of the products increases. But this influence is not so effective in more H2-rich (see Fig. 6(b)) and higher temperature environment, because the variation of the partial pressure caused by argon addition is inhibited at these conditions.
4 Validation
In order to validate the points of the thermodynamics, a series of relative experiments were performed in a quartz apparatus described in previous work [26]. The reagents and synthesis parameters are listed in Table 2.
Table 2 Reagents and synthesis parameters used for validation experiments

According to reaction (12), the reduction ratio of the pentachloride can be examined by measuring the amount of HCl in the exhaust. During the experiments, the exhaust gas was bubbled to pass through a glass container with enough distilled water, in order to fully absorb the HCl. After the experiments, the content of H+ in the solution was determined by the method of acid-base titration. The reduction ratio of the pentachloride (ηNbCl5) was calculated by
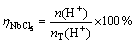 (13)
(13)
where n(H+) is the amount of the titration; nT(H+) is the theoretical amount in reaction (12).
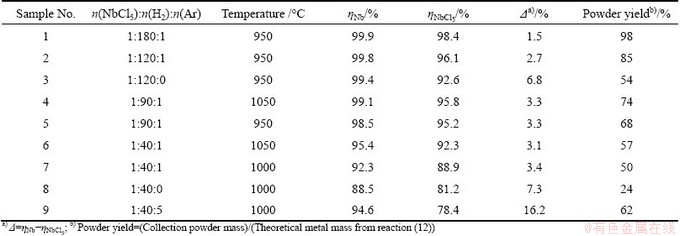
The difference (Δ) between ηNbCl5 and ηNb was employed to evaluate the results of thermodynamic. When the value of Δ is small enough, the thermodynamics is reliable. The comparison is shown in Table 3. As can be seen, the values of ηNbCl5 increase with the increase of hydrogen ratio and temperature, as well as that of ηNb. Most values of Δ are no more than 3.5%. Considering the errors of the titration measurement and a small amount of HCl(g) absorbed by the powder, the results of the experimental are in good agreement with those of the thermodynamics.
Furthermore, it is found that the loading gas can not only increase the reduction ratio of the pentachloride, but also improve the powder yield (samples 2 and 3, or 7 and 8 in Table 3). It is well known that the inert gas does not help the kinetics of a chemical reaction. But PRATSINIS and VEMURY [28] have pointed out that the mixing route of the reagents had a strong effect on the formation of powder in gas phases. In this work, the loading gas is hydrogen when no argon is used (samples 3 and 8), which means that the reagents have been mixed before the reaction happening. But when the loading gas is added (samples No. 2 and 7), the mixed route of the reagents is changed in time of the reaction happening. So, the powder yield should be improved.
However, at a constant temperature, when the argon ratios are 0, 1 and 5 (samples 7, 8 and 9 in Table 3), the powder yields are 24%, 50% and 62%, respectively, but ηNbCl5 decreases unexpectedly from 88.9% to 78.4%. Larger argon ratio means higher argon flux, which should shorten the mixture and retention time of the reagents in high temperature zone of the reactor and make reactions (5)-(11) react incompletely. This suggests that the argon ratio must be limited to an appropriate level to avoid the disadvantage of excess argon flux, although it is interesting that higher argon flux can increase the powder yield.
Table 3 Comparison of experimental data (ηNbCl5) and thermodynamic calculation value (ηNb) for hydrogen reduction by CVS process
The powder products were examined by X-ray diffraction (XRD) (Rigaku D/max-2400) and transmission electron microscopy (TEM) (JEOL JEM-100CX II). The results are shown in Fig. 7. It indicates that Nb nanopowder with average particle size of 30-50 nm is obtained at the selected conditions guided from the thermodynamics, with the ηNbCl5 of 96.1% and the powder yield of 85%.
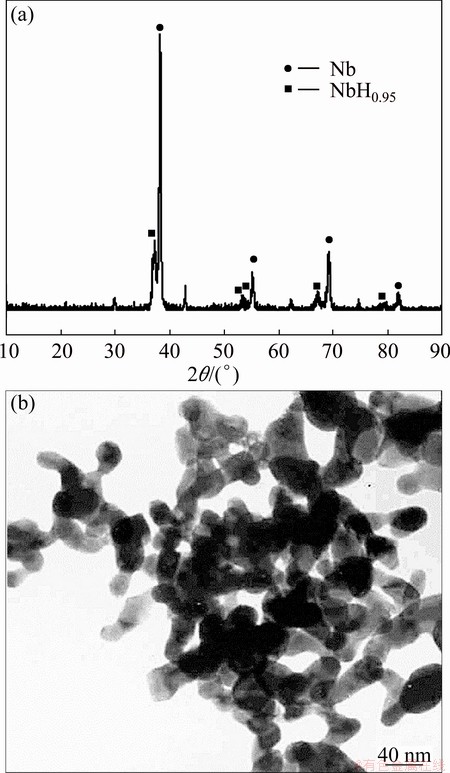
Fig. 7 XRD pattern (a) and TEM image (b) of Nb nanopowder synthesized at n(NbCl5):n(H2):n(Ar)= 1:120:1 and 950 °C
5 Conclusions
1) A detailed thermodynamic analysis by using FactSage software is performed for NbCl5-H2-Ar system. The reduction route from NbCl5(g) to Nb(s) is a stage-wise process with the formation of complex sub-chlorides. The reduction ratio of NbCl5 increases with the increase of hydrogen ratio and temperature. NbCl5 can be reduced to Nb(s) with the yield of Nb more than 99.9% at low hydrogen ratio (<180:1) and low temperature (<1050 °C).
2) The results of validation experiments are in good agreement with the thermodynamics. An appropriate amount of inert loading gas is benefit to increase both of the reduction ratio of NbCl5 and the powder yield, because the addition of the inert gas could change the partial pressure of the equilibrium system and the mixing route of the reagents.
3) The as-synthesized Nb nanopowder with the homogeneous particle size of 30-50 nm is obtained under n(NbCl5):n(H2):n(Ar)=1:120:1 and 950 °C, with the reduction ratio of NbCl5 of 96.1% and the powder yield of 85%.
References
[1] YOON J S, YANG J Y, LEE J M, HONG S J. Preparation of niobium powders for solid electrolyte capacitors through hunter process using metallothermic reduction method [J]. Materials Transactions, 2013, 54(1): 114-118.
[2] FREEMAN Y, ALAPATT G F, HARRELL W R, LESSNER P. Electrical characterization of high voltage polymer tantalum capacitors [J]. Journal of the Electrochemical Society A, 2012, 159(10): A1646-A1651.
[3] HE Ji-lin. New development of tantalum and niobium electronic materials [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(S1): s291-s300. (in Chinese)
[4] PURUSHOTHAM Y, RAVINDRANATH K, KUMAR A, GOVINDAIAH R, PRAKASH T L. Quality improvements in tantalum powder by automation of sodium reduction system [J]. International Journal of Refractory Metals and Hard Materials, 2009, 27(3): 571-576.
[5] JEONG E Y, JINSUB C. Electrochemical surface enlargement of a niobium foil for electrolytic capacitor applications [J]. Electrochemistry Communications, 2011, 13(3): 298-301.
[6] MIKI H, SHIMAMOTO Y, FURUKAWA R. Highly reliable MIS capacitors with plasma nitridation and doubled dielectric-constant tantalum pentoxide [J]. Electron Devices, IEEE Transactions on, 2005, 52(8): 1832-1838.
[7] QIU Y J, SMYTH D, KIMMEL J. The stabilization of niobium-based solid electrolyte capacitors [J]. Active and Passive Electronic Components, 2002, 25(2): 201-209.
[8] TRAINER M. The effects of neutron transmutations on the low-temperature dielectric properties of solid tantalum capacitors [J]. Materials Chemistry and Physics, 2003, 80(1): 264-268.
[9] HAAS H,  M. Tantalum capacitor anodes providing highest capacitances: Where are the limits? [C]//Proceedings- CARTS Europe 2011: the 24th Annual Passive Electronic Components Symposium. Nice, France: Holiday Inn Resort Nice, 2011: 1-6.
M. Tantalum capacitor anodes providing highest capacitances: Where are the limits? [C]//Proceedings- CARTS Europe 2011: the 24th Annual Passive Electronic Components Symposium. Nice, France: Holiday Inn Resort Nice, 2011: 1-6.
[10] YOO S H, SUDARSHAN T S, SETHURAM K, SUBHASH G, DOWDING R J. Consolidation and high strain rate mechanical behavior of nanocrystalline tantalum powder [J]. Nanostructured Materials, 1999, 12(1): 23-28.
[11] de BRITO R A, MEDEIROS F F P, GOMES U U, COSTA F A, SILVA A G P, ALVES C Jr. Production of tantalum by aluminothermic reduction in plasma reactor [J]. International Journal of Refractory Metals and Hard Materials, 2008, 26(5): 433-437.
[12] BABA M, ONO Y, SUZUKI R O. Tantalum and niobium powder preparation from their oxides by calciothermic reduction in the molten CaCl2 [J]. Journal of Physics and Chemistry of Solids, 2005, 66(2): 466-470.
[13] LI Jian, YI Dan-qing, WEN Jun-jie, ZHONG Hai-yun. Preparation of new electrolytic capacitor anode of niobium suboxide [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(6): 893-899. (in Chinese)
[14] YUAN B Y, OKABE T H. Niobium powder production by reducing electrochemically dissolved niobium ions in molten salt [J]. Journal of Alloys and Compounds, 2008, 454(1): 185-193.
[15] PARK I I, OKABE T H, WASEDA Y. Tantalum powder production by magnesiothermic reduction of TaCl5 through an electronically mediated reaction (EMR) [J]. Journal of Alloys and Compounds, 1998, 280(1): 265-272.
[16] ZHU H M, SADOWAY D R. Synthesis of nanoscale particles of Ta and Nb3Al by homogeneous reduction in liquid ammonia [J]. Journal of Materials Research, 2001, 16(1): 2544-2549.
[17] MA Chun-hong, ZHANG Wei-feng, HE Ji-lin, ZHU Hong-min. Synthesis and characterization of tantalum nitride nanopowder prepared through homogeneous reaction [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(S1): s556-s559.
[18] GRABIS J, MUNTER R, BLAGOVESHCHENSKIY Y, GORKUNOV V, YAMSHCHIKOV L. Plasmochemical process for the production of niobium and tantalum nanopowders [J]. Proceedings of the Estonian Academy of Sciences, 2012, 61(2): 137-145.
[19] PARK K Y, KIM H J, SUH Y J. Preparation of tantalum nanopowders through hydrogen reduction of TaCl5 vapor [J]. Powder Technology, 2007, 172(3): 144-148.
[20] KOENIG T, FISTER D. Fine-particle metal powders: US, 5407458 [P]. 1995-04-18.
[21] XU Y D, YAN X T. Chemical vapour deposition: An integrated engineering design for advanced materials [M]. London: Springer-Verlag, 2010: 136.
[22] LIU Q M, ZHANG L T, LIU J, WANG Y G. Thermodynamic study on codeposition of ZrC-SiC from MTS-ZrCl4-CH4-H2 [J]. Inorganic Materials, 2010, 46(10): 1090-1095.
[23] ZHAN Jing, HE Yue-hui, ZHOU Di-fei, ZHANG Chuan-fu. Thermodynamic analysis on synthesis of fibrous Ni-Co alloys precursor and Ni/Co ratio control [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 1141-1148.
[24] PEREZ-LABRA M, ROMERO-SERRANO A, HERNANDEZ- RAMIREZ A, ALMAGUER-GUZMAN I, BENAVIDES-PEREZ R. Effect of CaO/SiO2 and Fe/SiO2 ratios on phase equilibria in PbO-ZnO-CaO-SiO2-“Fe2O3” system in air [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(3): 665-674.
[25] DENG J, CHENG L, HONG Z, SU K, ZHANG L. Thermodynamics of the production of condensed phases in the chemical vapor deposition process of zirconium diboride with ZrCl4-BCl3-H2 precursors [J]. Thin Solid Films, 2012, 520(6): 2331-2335.
[26] CAO Zhan-min, QIAO Zhi-yu, ZHU Jun, ZHU Hong-min. Thermodynamic simulation of hydrogen reduction of NbCl5 to produce Nb metal powder [J]. Journal of University of Science and Technology Beijing, 2008, 30(6): 640-643. (in Chinese)
[27] BALE C W,  E, CHARTRAND P, DECTEROV S A, ERIKSSON G, HACK K, JUNG I H, KANG Y B,
E, CHARTRAND P, DECTEROV S A, ERIKSSON G, HACK K, JUNG I H, KANG Y B,  J, PELTON A D, ROBELIN C, PETERSEN S. FactSage thermochemical software and databases—Recent developments [J]. Calphad, 2009, 33(2): 295-311.
J, PELTON A D, ROBELIN C, PETERSEN S. FactSage thermochemical software and databases—Recent developments [J]. Calphad, 2009, 33(2): 295-311.
[28] PRATSINIS S E, VEMURY S. Particle formation in gases: A review [J]. Powder Technology, 1996, 88(3): 267-273.
NbCl5-H2-Ar体系化学气相合成纳米Nb粉的热力学
朱 骏,黄 凯,侯军刚,朱鸿民
北京科技大学 冶金与生态工程学院,稀贵金属绿色回收与提取北京市重点实验室,北京 100083
摘 要:采用FactSage软件,对NbCl5-H2-Ar体系化学气相合成纳米金属Nb粉的热力学进行研究并对热力学研究结果进行实验验证。结果表明:在标准大气压下,NbCl5(g)被氢气还原为Nb(s)是一个多步骤反应过程,其间产生大量中间产物(低价氯化物);该反应过程可控制在较低氢气比率(n(NbCl5):n(H2)<1:180)和较低温度(<1050 °C)下完全进行;适量的惰性载流气体可同时提高NbCl5转化率和粉末收得率。根据热力学计算得到的合成条件为n(NbCl5): n(H2):n(Ar)=1:120:1以及950 °C,实验成功制得平均粒径为30~50 nm且粒度分布均匀的纳米金属Nb粉,相应的NbCl5转化率达到 96.1%,粉末收得率为85%。
关键词:NbCl5-H2-Ar体系;化学气相合成;热力学;Nb纳米粉末;FactSage软件
(Edited by Yun-bin HE)
Foundation item: Project (51102015) supported by the National Natural Science Foundation of China
Corresponding author: Hong-min ZHU; Tel: +86-10-62334775; E-mail: hzhu@metall.ustb.edu.cn
DOI: 10.1016/S1003-6326(14)63560-3
Abstract: Thermodynamics for chemical vapor synthesis (CVS) of Nb nanopowder in NbCl5-H2-Ar system was investigated by using FactSage software. The validation experiments were conducted to confirm the thermodynamics points. The results indicate that under the atmospheric pressure, the reduction approach from NbCl5(g) to Nb(s) is a stage-wise process with the formation of complex sub-chlorides, and is controllable at low hydrogen ratio (mole ratio of n(NbCl5):n(H2)<1:180) and low temperature (<1050 °C). Furthermore, a reasonable amount of inert loading gas is favorable to increase the reduction ratio of NbCl5 and the powder yield. The as-synthesized Nb nanopowder with the homogeneous size of 30-50 nm and the powder yield of 85% (mass fraction) is obtained by the CVS process under n(NbCl5):n(H2):n(Ar)=1:120:1 and 950 °C with the NbCl5 reduction rate of 96.1%.


