Trans. Nonferrous Met. Soc. China 22(2012) 3108-3112
Effect of Na2O on formation of calcium aluminates in CaO-Al2O3-SiO2 system
YU Hai-yan1, PAN Xiao-lin1, WANG Bo1, 2, ZHANG Wu1, SUN Hui-lan2, BI Shi-wen1
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
2. School of Materials Science and Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, China
Received 12 December 2011; accepted 21 May 2012
Abstract:
The formation characteristics of calcium aluminates in the CaO-Al2O3-SiO2 system with sodium oxide was investigated by XRD, SEM-EDS and DSC-TG technologies. The main phases in the clinker after sintering at 1350 °C are 12CaO·7Al2O3, 2CaO·Al2O3·SiO2 and 2CaO·SiO2 when the mass ratio of Al2O3 to SiO2 is 3.0 and the molar ratio of CaO to Al2O3 is 1.0. The proportion of 12CaO·7Al2O3 increases with the increase of Na2O addition when the molar ratio of Na2O to Al2O3 is from 0 to 0.4, while the proportion of 2CaO·Al2O3·SiO2 decreases with the increase of Na2O addition. Na2O forms solid solution in 12CaO·7Al2O3, which increases the volume of elementary cell of 12CaO·7Al2O3. The formation temperature of 12CaO·7Al2O3 is decreased by 30 °C when the molar ratio of Na2O to Al2O3 increases from 0 to 0.4 determined by DSC. The alumina leaching property of clinker increases obviously with the increase of Na2O addition.
Key words:
CaO-Al2O3-SiO2 system; Na2O; Al2O3; 12CaO·7Al2O3; sintering; leaching;
1 Introduction
CaO-Al2O3-SiO2 (C-A-S) ternary system is of considerable importance in oxide ceramics, cement chemistry, metallurgical slags and geochemistry [1-3]. Meanwhile, the C-A-S system plays an important role in the production of alumina industry by the sintering process in China [4]. The phase diagram of the C-A-S system can be seen elsewhere, and the thermodynamic analyses of the ternary system have been performed by PELTON et al [5] using a quasichemical model, by WANG et al [6] using an ionic sublattice model and by FABRICHNAYA and NERAD [7] using a molecular model.
Meanwhile, the formation characteristics of calcium aluminates has been widely investigated [8,9], such as 3CaO·Al2O3 (C3A) [10], 12CaO·7Al2O3 (C12A7) [11], CaO·Al2O3 (CA) [12], CaO·2Al2O3 (CA2) [13] and CaO·6Al2O3 (CA6) [14]. GRZESZCZYK [15] and OSTROWSKI and FLAZNY [16] studied the solid solutions of calcium aluminates with sodium ion formed at high temperature in CaO-Al2O3 binary system, and sodium ions can be built into the calcium aluminate system. Furthermore, SUN et al [17] found that addition of Na2O during the sintering process can promote the alumina leaching property of calcium aluminate slag, but the mechanism was not proposed.
In the C-A-S system, the phases in the sintered clinker referred to alumina production consist of calcium aluminates, 2CaO·SiO2 (C2S) and 2CaO·Al2O3·SiO2 (C2AS). C2S comprises several different types, such as α′-C2S, β-C2S, γ-C2S. Previous studies [18] indicated that C12A7 has better alumina leaching properties than other calcium aluminates in sodium carbonate solution, while C2AS is difficult to be extracted to solution. C2S is very stable in sodium carbonate solution. For the great role of sodium oxide during the sintering process, it is necessary to reveal the reaction mechanisms in the C-A-S system in the presence of sodium ions. Therefore, the aim of this work is to study the effect of sodium oxide on the sintering characteristics and leaching property of calcium aluminates in the C-A-S system with low mass ratios of Al2O3 to SiO2 and CaO to Al2O3.
2 Experimental
Analytically pure reactants were used in the present work and the calculated oxide ratios for sintering process are listed in Table 1. CaO and Na2O were added in the forms of CaCO3 and Na2CO3, respectively. The mass ratio of Al2O3 to SiO2 (A/S) is 3.0, and the molar ratio of CaO to Al2O3 (C/A) is 1.0 (the residual CaO subtracted the composition to form 2CaO·SiO2). The molar ratio of Na2O to Al2O3 (N/A) ranges from 0 to 0.4 as presented in Table 1. The mixtures were milled in a ball mill for 3 h, and then sintered at 1350 °C for 1 h in a MoSi2 resistance furnace followed by cooling in the furnace.
Table 1 Oxide ratios of samples for sintering
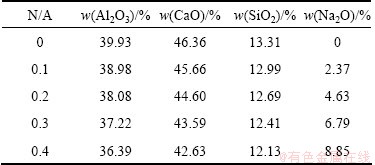
The sintered clinkers were leached at 75 °C for 30 min in sodium carbonate solution. The concentration of sodium carbonate solution (in the form of Na2O) is 80 g/L. The liquid-to-solid ratio of sodium carbonate solution to clinker for leaching is 10. The leached slurry was filtrated using a Buchner funnel. The concentration of caustic alkali (NK), total alkali (NT) and Al2O3 (AO) in the filter liquor were determined by the volumetric method, while the filter residue was washed carefully and dried for chemical analysis. The alumina leaching rate is calculated according to the following formula:
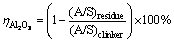 (1)
(1)
where (A/S)residue and (A/S)clinker are the mass ratios of Al2O3 to SiO2 in the leached residue and sintered clinker, respectively.
The contents of Al2O3, SiO2 and Na2O in samples and filtrate were analyzed by X-ray fluorescence (XRF, ZSX100e). Phase components of the clinker were identified by X-ray diffraction (PANalytical PW3040/60). SEM (SHIMADZU SSX-550) and EDS (DX-4) were used for microstructure and component analysis. Simultaneously recorded studies of differential scanning calorimetry (DSC) and thermogravimetric analysis were carried out using a NETZSCH STA409C/CD simultaneous thermal analyzer in a dynamic Ar atmosphere. The samples with the N/A ratios of 0 and 0.4 in Table 1 were selected. The samples were heated up to 1500 °C at a rate of 10 °C/min.
3 Results and discussion
3.1 Phase composition characteristics
The XRD patterns of sintered clinkers at different N/A ratios are shown in Fig. 1. When N/A=0.1, the clinker contains C2AS, C12A7, β-C2S, γ-C2S as well as some CA and CA2. As the N/A ratio increases to 0.2, the phase composition and content of clinker are different. The content of C12A7 increases, while the content of C2AS decreases. Meanwhile, CA2 disappears, and β-C2S is formed during the sintering process. When N/A=0.4, most of the clinker is C12A7, and the content of C2AS is very low. Furthermore, both CA and CA2 do not exist, while β-C2S and γ-C2S coexist in the clinker. It can be concluded that as the molar ratio of Na2O to Al2O3 increases from 0 to 0.4, the proportion of C12A7 in the clinker increases with the increase of N/A ratio when A/S=3.0 and C/A=1.0.
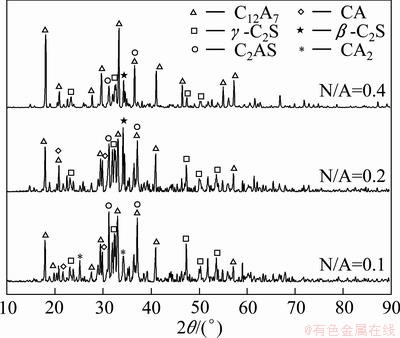
Fig. 1 XRD patterns of sintered clinkers at different N/A ratios
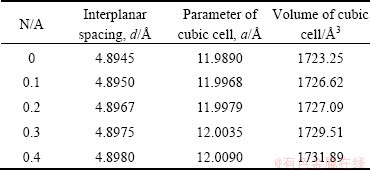
The crystal structure of C12A7 also changes as the N/A ratio increases. The interplanar spacing corresponding to the strongest characteristic peak (2θ=18.109°) of C12A7 and the parameter of cubic cell as well as its volume at different N/A were calculated, as listed in Table 2. Both the interplanar spacing and the volume of elementary cell of C12A7 increase with the increase of N/A, indicating that Na2O forms solid solution in C12A7, which is consistent with Ostrowski’s results [16]. Therefore, the solid solution of Na2O in C12A7 is beneficial to the formation of C12A7.
Table 2 Effect of N/A ratio on lattice parameters of C12A7
When N/A=0.4, the representative microstructure of sintered clinker is shown in Fig. 2. The morphology of particles is massive, and the particles can be divided to two kinds by the size. Most of the particles have a larger size, and are usually several micrometers, even larger than 10 μm. The other particles are relatively small, and are usually smaller than 1 μm. These particles are adsorbed on the surface of the larger particles.
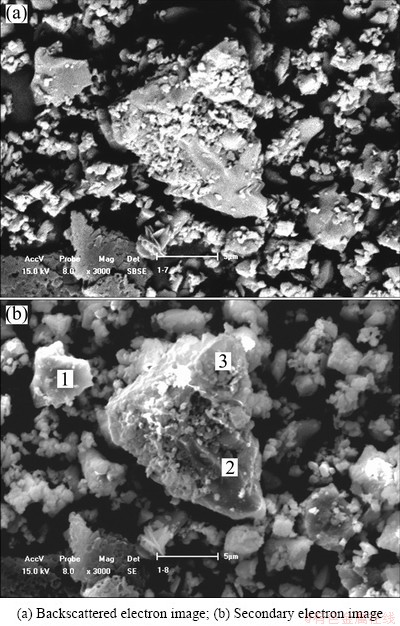
Fig. 2 SEM images of sintered clinker when N/A=0.4
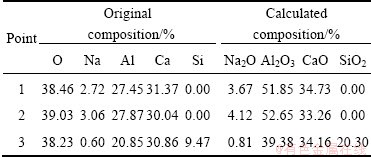
The compositions of larger particles (points 1 and 2 in Fig. 2(b)) and smaller particles (point 3) determined by EDS analysis are listed in the left of Table 3, and the corresponding compositions of oxides are calculated in the right of Table 3. No SiO2 is discerned in the large particles, and the compositions are similar to of C12A7. Therefore, the larger particles are C12A7. The composition ratio of CaO to SiO2 in the smaller particles is close to 2, indicating that the smaller particles are C2S.
Furthermore, as presented in Table 3, the composition of Na2O in the larger particles is about 4%, while the composition of Na2O in the smaller particles is below 1%. Because no compound containing Na2O was found by XRD analysis, Na2O must be solid dissolved in the crystal lattice of C12A7, which is consistent with the calculated results of lattice parameters of C12A7, as presented in Table 2.
Table 3 Phase compositions of clinker when N/A=0.4 corresponding to Fig. 2(b)
3.2 Thermal analysis
The DSC heating and TG curves of both samples with the N/A ratios of 0 and 0.4 are shown in Fig. 3. Two strong endothermic peaks with a large gravity decrease at 798 °C exist in both curves, indicating that they relate to the decomposition of CaCO3. A small endothermic peak with a small gravity decrease at 864 °C in Fig. 3(b) indicates that it relates to the melting of Na2CO3. C2S is formed below 1300 °C [4], occurring at the endothermic peaks between 1000 °C and 1300 °C for both samples. As shown in Fig. 1, C12A7 is the main phase in the clinker when the N/A ratio is 0.4, and therefore, the large endothermic peak at 1360 °C in Fig. 3(b) relates to the formation of C12A7. As an intermediate phase, C2AS is formed before C12A7 in the CaO-Al2O3-SiO2 system [4]. Therefore, the endothermic peak at 1342 °C should be associated with the formation of C2AS, and the endothermic peak at 1390 °C represents the formation of C12A7. Addition of Na2O can not only promote the formation of C12A7 and inhibit the formation of C2AS, but also decrease the formation temperature of C12A7 by 30 °C.
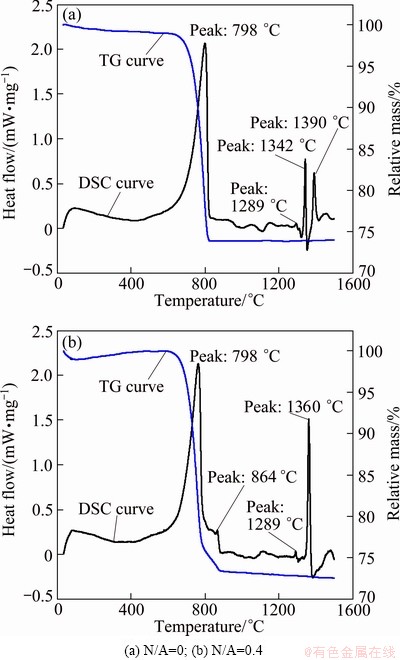
Fig. 3 DSC-TG curves of different mixtures during heating process
3.3 Alumina leaching property
The leaching results of clinkers at different N/A ratios in sodium carbonate solution are listed in Table 4, and alumina leaching rates are calculated by formula (1) as shown in Fig. 4. The reaction of calcium aluminates with sodium carbonate solution is shown in formula (2).
xCaO·yAl2O3+xNa2CO3+(x+3y)H2O→xCaCO3↓+2yNaAl(OH)4+(2x-2y)NaOH (2)
The alumina leaching rate increases obviously with the increase of N/A ratio. It is 86.91% when N/A=0.4, which is much higher than that when N/A=0 by 43.52%. Therefore, the addition of Na2O can greatly improve the leaching properties of sintered clinker.
Table 4 Leaching results of clinkers at different N/A ratios in sodium carbonate solution
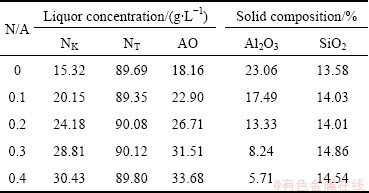
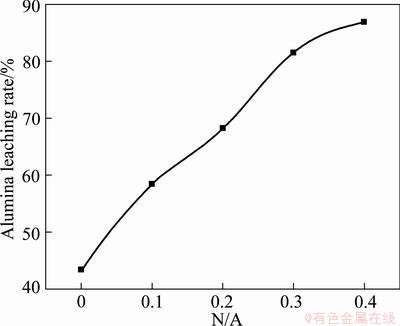
Fig. 4 Effect of N/A ratio on alumina leaching rate of clinkers
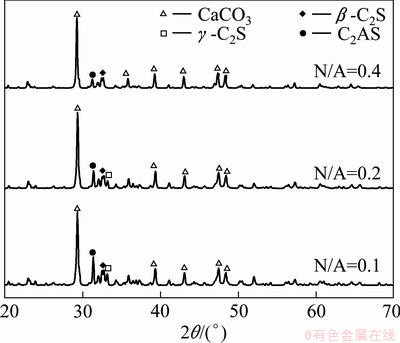
Fig. 5 XRD patterns of leached residues at different N/A ratios
The XRD patterns of leached residues are shown in Fig. 5. The main phases are CaCO3, C2S and C2AS. No C12A7 is found in the leached residues, indicating that all Al2O3 in C12A7 is extracted into the solution during the leaching process.
4 Conclusions
1) Na2O promotes the formation of C12A7 and inhibits the formation of C2AS sintered at 1350 °C when A/S=3.0 and C/A=1.0 in the CaO-Al2O3-SiO2 system.
2) Na2O forms solid solution in C12A7 which increases the volume of elementary cell of C12A7.
3) The formation temperature of C12A7 is decreased by 30 °C when the molar ratio of Na2O to Al2O3 increases from 0 to 0.4, and the leaching properties of the sintered clinker are greatly increased.
References
[1] BANIJAMALI S, EFTEKHARI Y B, REZAIE H R, MARGHUSSIAN V K. Crystallization and sintering characteristics of CaO-Al2O3-SiO2 glasses in the presence of TiO2, CaF2 and ZrO2 [J]. Thermochimica Acta, 2009, 488(1-2): 60-65.
[2] SINGH V K. Sintering of calcium aluminate mixes [J]. British Ceramic Transactions, 1999, 98(4): 187-191.
[3] MELLER N, HALL C, PHIPPS J S. A new phase diagram for the CaO-Al2O3-SiO2-H2O hydroceramic system at 200 °C [J]. Materials Research Bulletin, 2005, 40(5): 715-723.
[4] BI Shi-wen, YU Hai-yan. Production technology of alumina [M]. Beijing: Chemical Industry Press, 2006: 225-230. (in Chinese)
[5] PELTON A D, WU P, ERIKSSON G. Critical evaluation and optimization of the phase diagrams and thermodynamic properties of oxide systems [R]. Final Report, Center for Research in Computational Thermochemistry, Ecole Polytechnique. Montreal, Canada, 1984: 1-14.
[6] WANG X, HILLERT M, SUNDMAN B. A thermodynamic evaluation of the Al2O3-CaO-SiO2 system [R]. TRITA-MAC-0407, Royal Institute of Technology. Stockholm, 1989: 1-19.
[7] FABRICHNAYA O B,  I. Thermodynamic properties of liquid phase in the CaO·SiO2-CaO·Al2O3·2SiO2- 2CaO·Al2O3·SiO2 system [J]. Journal of the European Ceramic Society, 2000, 20(4): 505-515.
I. Thermodynamic properties of liquid phase in the CaO·SiO2-CaO·Al2O3·2SiO2- 2CaO·Al2O3·SiO2 system [J]. Journal of the European Ceramic Society, 2000, 20(4): 505-515.
[8] HALLSTEDL B. Assessment of the CaO-Al2O3 System [J]. Journal of the American Ceramic Society, 1990, 73(1): 15-23.
[9] SINGH V K, ALI M M, MANDAL U K. Formation kinetics of calcium aluminates [J]. Journal of the American Ceramic Society, 1990, 73(4): 872-876.
[10] MOHAMED B M, SHARP J H. Kinetics and mechanism of formation of tricalcium aluminate, Ca3Al2O6 [J]. Thermochimica Acta, 2002, 388(1-2): 105-114.
[11] YI H C,  J Y. Preparation of calcium aluminate matrix composites by combustion synthesis [J]. Journal of Materials Science, 2002, 37(21): 4537-4543.
J Y. Preparation of calcium aluminate matrix composites by combustion synthesis [J]. Journal of Materials Science, 2002, 37(21): 4537-4543.
[12] CHEN Guo-hua. Mechanical activation of calcium aluminate formation from CaCO3-Al2O3 mixtures [J]. Journal of Alloys and Compounds, 2006, 416(1-2): 279-283.
[13] IFTEKHAR S, GRINS J, SVENSSON G,  J, JARMAR T, BOTTON G A, ANDREI C M, ENGQVIST H. Phase formation of CaAl2O4 from CaCO3-Al2O3 powder mixtures [J]. Journal of the European Ceramic Society, 2008, 28(4): 747-756.
J, JARMAR T, BOTTON G A, ANDREI C M, ENGQVIST H. Phase formation of CaAl2O4 from CaCO3-Al2O3 powder mixtures [J]. Journal of the European Ceramic Society, 2008, 28(4): 747-756.
[14] RIDWAN I, ASMI D. The use of rietveld technique to study phase composition and developments of calcium aluminate [J]. AIP Conference Proceedings, 2008, 989: 180-183.
[15] GRZESZCZYK S. Solid solutions of C3A-Na2O in C12A7 [J]. Cement and Concrete Research, 1986, 16(6): 798-804.
[16] OSTROWSKI C, ELAZNY J. Solid solutions of calcium aluminates C3A, C12A7 and CA with sodium oxide [J]. Journal of Thermal Analysis and Calorimetry, 2004, 75(3): 867-885.
[17] SUN Hui-lan, WANG Bo, YU Hai-yan, BI Shi-wen, TU Gan-feng. Effect of Na2O on alumina leaching and self-disintegrating property of calcium aluminate slag [J]. Light Metals, 2010: 29-32.
[18] CHOU K S, BURNET G. Formation of calcium aluminates in the lime-sinter process. Part II. Kinetic study [J]. Cement and Concrete Research, 1981, 11(2): 167-174.
氧化钠对CaO-Al2O3-SiO2三元系铝酸钙形成规律的影响
于海燕1,潘晓林1,王 波1, 2,张 武1,孙会兰2,毕诗文1
1. 东北大学 材料与冶金学院,沈阳 110004;
2. 河北科技大学 材料科学与工程学院,石家庄 050018
摘 要:利用XRD、SEM-EDS和DSC-TG技术研究了添加Na2O的CaO-Al2O3-SiO2体系中铝酸钙的形成规律。结果表明,当Al2O3与SiO2的质量比为3.0、CaO与Al2O3的摩尔比为1.0时,在1350 °C烧结后的熟料主要由12CaO·7Al2O3、2CaO·Al2O3·SiO2和2CaO·SiO2组成。熟料中12CaO·7Al2O3的含量随着Na2O的增加而增加,2CaO·Al2O3·SiO2的含量随着Na2O的增加而降低。Na2O在12CaO·7Al2O3中形成固溶体,增加了其单位晶胞体积。DSC分析表明,Na2O不仅促进了12CaO·7Al2O3的形成,而且使C12A7的形成温度降低了30 °C。烧结熟料中的氧化铝溶出性能随着Na2O的增加而大幅度提高。
关键词:CaO-Al2O3-SiO2系;Na2O;Al2O3;12CaO·7Al2O3;烧结;溶出
(Edited by YANG Hua)
Foundation item: Projects (51174054, 51104041) supported by the National Natural Science Foundation of China
Corresponding author: YU Hai-yan; Tel: +86-24-83686460; E-mail: yuhy@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61578-1
Abstract: The formation characteristics of calcium aluminates in the CaO-Al2O3-SiO2 system with sodium oxide was investigated by XRD, SEM-EDS and DSC-TG technologies. The main phases in the clinker after sintering at 1350 °C are 12CaO·7Al2O3, 2CaO·Al2O3·SiO2 and 2CaO·SiO2 when the mass ratio of Al2O3 to SiO2 is 3.0 and the molar ratio of CaO to Al2O3 is 1.0. The proportion of 12CaO·7Al2O3 increases with the increase of Na2O addition when the molar ratio of Na2O to Al2O3 is from 0 to 0.4, while the proportion of 2CaO·Al2O3·SiO2 decreases with the increase of Na2O addition. Na2O forms solid solution in 12CaO·7Al2O3, which increases the volume of elementary cell of 12CaO·7Al2O3. The formation temperature of 12CaO·7Al2O3 is decreased by 30 °C when the molar ratio of Na2O to Al2O3 increases from 0 to 0.4 determined by DSC. The alumina leaching property of clinker increases obviously with the increase of Na2O addition.


