Trans. Nonferrous Met. Soc. China 27(2017) 2474-2480
Effect of grinding with sulfur on surface properties and floatability of three nonferrous metal oxides
Zhao LI, Min CHEN, Peng-wu HUANG, Qi-wu ZHANG, Shao-xian SONG
School of Resources and Environmental Engineering, Wuhan University of Technology, Wuhan 430070, China
Received 1 July 2016; accepted 9 October 2016
Abstract:
Co-grinding three nonferrous metal oxides (CuO, PbO and ZnO) with element sulphur under mild conditions and flotation of the ground samples were conducted to investigate the surface properties and floatability of the oxides. Phase transition, morphological features, electrochemical properties and surface chemical compositions of ground samples were studied. The results show that the floatability of CuO is improved after grinding with sulfur, by the formation of surface layer with properties similar to CuS due to the Cu—S bonding. The floatability of PbO is deteriorated after mechanochemical processing due to surface carbonation and the formation of PbS and PbSO4 by disproportionation reaction with sulfur. ZnO shows no evident response to mechanochemical sulfidation.
Key words:
grinding; nonferrous metal oxides; flotation; sulfur; recycling;
1 Introduction
Nonferrous metals, such as copper, lead and zinc, are mainly extracted from natural sulfide ores which are the primary nonferrous sources and steadily becoming depleted [1,2]. Meanwhile, it is reported that one third outputs of the nonferrous metals in Japan have been discharged in various types of waste. The utilization of low-grade oxidized type of ores and recycling of these metals from high metal-contained wastes, in which metals also exist commonly in an oxidized type rather than sulfide type, are of urgent demand, from the point of environmental resources preservation and sustainable development [3-9].
One common method for processing nonferrous metal minerals is flotation, and the flotation of oxidized minerals is more difficult than that of the corresponding sulfide minerals [10-12], due to the hydrophilicity surface and electrostatic repulsion between negatively charged surfaces of bubble and solids. Many methods have been reported to deal with nonferrous metal oxides, such as alkaline leaching [13,14] and sulfidation roasting [15,16], but without enough satisfactory results. An improved flotation recovery of nonferrous oxides can be obtained by adding Na2S solution into the pulp to modify the mineral surface with a layer of properties similar to that of natural sulfides from the adsorption of sulfide ions [17,18]. However, it is not easy to control the dosage of Na2S solution to obtain high recovery, which may be easily worsened by either an inadequate or excess initial Na2S concentration, not to say the environmental burden of treating the wastewater with sulfide ions.
Mechanochemical process has been widely used in many fields, such as syntheses of functional materials [19-22], hydrometallurgical process [23-25] and bio-pharmaceuticals [26,27]. Basic researches have been conducted on the transformation of oxides into sulfides by co-grinding with sulfur and reducing agents such as iron or aluminum powders [28-31], allowing the use of cheap sample of element sulfur to replace sulfides as sulfidizing agent. The use of element sulfur may also help to avoid the need to treat the wastewater with sulfide ions. Mechanochemical process may offer a relatively easy pathway for processing the oxides; however, the heavy agglomeration from the mechanochemical operation did not permit an easy application of the current flotation technique on the mechanochemically synthesized sulfides to obtain satisfied recovery. Changes are required to regulate the grinding operation without the formation of heavy agglomeration to match flotation operation to raise the floatability of the samples.
As part of basic researches to develop new process to exploit oxidized type of minerals as well as related solid wastes, pretreatment by co-grinding nonferrous metal oxides with element sulfur under relatively mild conditions without triggering obvious solid state reaction and heavy agglomeration is proposed as a key operation to improve the floatability of the oxide samples under typical flotation conditions. In this work, the obtained fundamental results are reported regarding the changes in the floatability of CuO, PbO and ZnO before and after co-grinding with sulfur. Quartz SiO2 has been applied as a reference sample. Changes in physico-chemical properties of these oxides after grinding have been investigated to understand the reasons for the changes in floatability.
2 Experimental
2.1 Materials
All the chemical reagents were of analytical grade from Sinopharm Chemical Reagent Co., Ltd., China and used as received. Copper oxide (CuO), lead oxide (PbO), zinc oxide (ZnO), quartz (SiO2) and sulfur (S) were used in the mechanochemical sulfidization. Element sulfur was used as the sulfidizer. Conventional flotation agents, butyl xanthate and pine camphor oil were used in flotation test, as collector and froth agent, respectively.
2.2 Procedure and apparatus
Metal oxides were mixed with sulfur at a mass ratio of 20:1, without any other additive. A planetary ball mill (QM-3SP4, Nanjing, China) was used for grinding of the mixtures. 5.25 g starting compounds were put into a stainless steel pot (500 cm3 inner volume) with 140 g stainless steel balls (7 balls with 17 mm in diameter) and subjected to grinding in air at different rotational speeds for 20 min. All grinding operation was conducted under ambient atmosphere, as a simulation to the actual industry situation.
A conventional rougher flotation operation was conducted to investigate the changes in floatability of the as-prepared samples. 5.25 g as-ground samples were employed in flotation experiment. Flotation tests were performed with a laboratory scale flotation machine (XFGC2 5-35 g, Jilin, China) under a constant pulp content of 10.5% with laboratory deionized water at room temperature. After the pulp was agitated for 5 min, collector in a concentration of 0.2 g/L was added and conditioned for another 5 min. Then, the foaming agent in a concentration of 0.05 g/L was added and conditioned for further 2 min. The froth was scraped by hand every 10 s and continued for totally 3 min, according to a plateau level of conventional rougher flotation procedure. The flotation efficiency was evaluated on the basis of the recovery of metal oxides.
2.3 Characterizations
The crystallographic compositions of the ground products were characterized with X-ray diffractometer (XRD, RIGAKU, D/MAX-RB, Japan). The morphological change and the superficial chemical composition of the samples after ball milling were observed with a scanning microscope (SEM-EDS, JEOL Ltd, JSM-5610LV, Japan). A Fourier transformation infrared spectrometer (FTIR, Thermo Nicolet, Nexus, USA) was used to analyze the ground samples with a conventional KBr method. A Raman spectrometer (Raman, RENISHAW, INVIA, UK) was used to analyze the property change of the ground sample. Power- compensation differential scanning analysis (DSC, Perkin Elmer, Pyris, USA) was carried out under N2 atmosphere heating from room temperature to 700 °C at 10 °C/min to assess the adsorption of moisture and carbon dioxide on the sample surface. Laser particle size analysis (Mastersizer 2000, Malvern, UK) was used to investigate the size distributions of ground samples. Before operating, 0.01 g of sample was dipped in 100 mL deionized water and then dispersed for 5 min using an ultrasonic disperser. The adjustment of pH value was based on the addition of NaOH solution and diluted hydrochloric acid. A Zeta-potential measurement (Zetasizer Nano, Malvern, UK) was performed to investigate the surface electrochemical properties of samples before and after grinding.
3 Results and discussion
3.1 Phase transition of three oxides during grinding with sulfur
Figure 1 shows the XRD patterns of three oxide samples ground at 500 r/min for 20 min, respectively. In CuO and ZnO cases, all the peaks for the ground mixtures are the same as those of the initial materials except for their heights. This implies that such a mild grinding cannot cause the formation of metal sulfide. The clear peaks of sulfur in ZnO pattern testify the uneven dispersion and feeble binding of sulfur on ZnO surface after grinding. Differently, the broad and weak peaks of sulfur in CuO case indicate the more uniform dispersion of sulfur on the surface of CuO.
Compared with above two cases, the XRD peaks of PbO are obviously of weak intensity and broad shape, indicating that its crystallinity has changed in some sense. Simultaneously, there is no initial sulfur but slight PbS in the pattern of PbO, confirming a kind reaction with PbO, represented as Eq. (1):
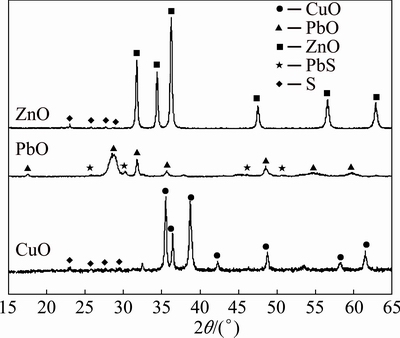
Fig. 1 XRD patterns of mixtures of 5 g MeO (Me = Cu, Pb and Zn) and 5% sulfur ground at 500 r/mom for 20 min
4PbO+4S→PbSO4+3PbS (1)
This disproportionation reaction, investigated in our previous study [29], is the key mechanism to explain the mechanochemical reaction between lead oxide and sulfur. The disproportionation reaction between nonferrous oxide and element sulfur can be triggered under such a mild mechanochemical condition and only in the case of PbO, leading to a direct solid state reaction between PbO and S. Such a reaction phenomenon cannot be detected in the cases of CuO and ZnO.
3.2 Morphological features of ground particles
SEM microphotographs of mechanochemically processed CuO particles without and with sulfur are given in Fig. 2. It is seen that particles of two samples are about 5-10 μm in size on average and the surface of these particles are smooth. The morphology of two ground samples is almost identical to original sample, although not shown here, and completely different from the observed heavy agglomeration of prime nano-range particles by means of mechanochemical solid-state reaction [29]. Meanwhile, since surface porosity plays a very important part in the mechanochemical reaction [32], agglomeration of fine particles on surface may reduce diffusion efficiency and inhibit flotation. Therefore, from the aspects of morphology and diffusivity, grinding under a mild condition is expected to allow sulfur coating to move towards the surface of oxide minerals and avoid agglomeration simultaneously. The obtained samples are in morphology feasible for flotation without the need of specific dispersion treatment in advance.
Since flotation is essential to feed particle size, the particle size distributions of three ground oxide samples, which were prepared under the grinding conditions with maximum flotation recovery, were characterized and the results are shown in Fig. 3. The sizes of particles are rather uniform with an average hydrodynamic diameter over 10 μm, within the handling scope of flotation. Meanwhile, the narrow particle size distributions imply the uniform crystallographic characteristics of ground samples, which are similar to those of initial materials before grinding.

Fig. 2 SEM photographs of ground samples of CuO without (a) and with (b) sulfur
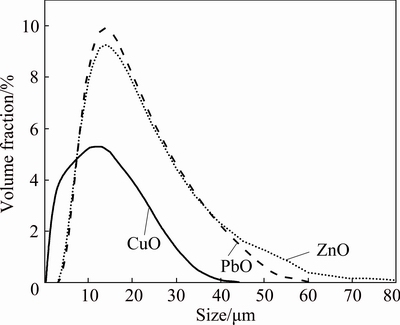
Fig. 3 Particle size distributions of three ground metal oxides
3.3 Flotation properties of different oxides after mechanochemical processing
Figure 4 shows the changes in flotation recovery of different oxides with rotational speed of grinding for 20 min. It is observed that CuO and SiO2 are initially unfloatable, different from PbO and ZnO, represented as recovery at 0 r/min. The recovery of CuO increases with an increase in rotational speed and reaches about 80% at 400 r/min, indicating the contribution of grinding operation to the improvement in the floatability of CuO sample. The constant low flotation recovery of SiO2 in the whole process can be interpreted as the weak physical bonding of sulfur on SiO2 particle surfaces, instead of mechanical flotation or impurities caused by prolonging time of grinding. The recovery of ZnO responds slightly to the increase of grinding intensity, indicating that the grinding operation has little impact on the floatability of ZnO.
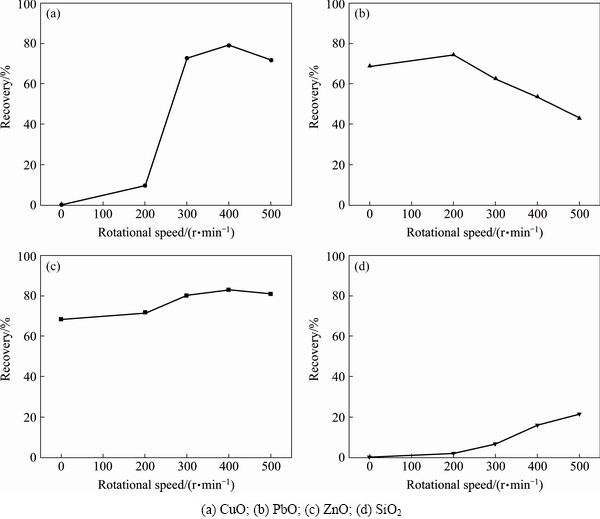
Fig. 4 Changes in recovery of various oxides with grinding rotational speed
Diametrically different from the cases of CuO and ZnO, the flotation recovery of PbO decreases gradually. This means that grinding operation expresses a negative effect on the flotation efficiency of PbO. In addition to the previously mentioned disproportionated reaction, which will generate lead sulfate, other changes in the surface properties of lead oxide particles occurring during grinding process may also deteriorate the floatability.
3.4 Electrochemical properties of mechanochemically processed particles
Figure 5 shows the changes in Zeta potentials of both CuO and PbO samples before and after grinding at 500 r/min for 20 min, at the pH corresponding to the flotation process.
In the case of CuO, compared with the original sample, the sample co-ground with sulfur exhibits a Zeta potential toward a positive movement more than 20 mV.
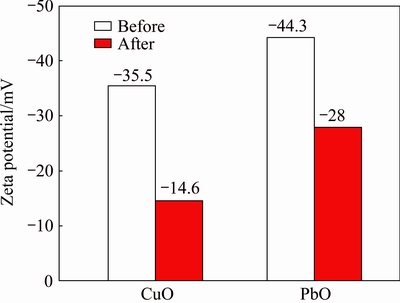
Fig. 5 Zeta potentials of CuO and PbO before and after grinding with sulfur
The chemically modified surfaces transform copper oxide into sulfide-like and the potential changes into positive. As part of the reason, the more positive surfaces may improve an easy adsorption of xanthate and the flotation recovery promotion will be obtained as a result. Similar to CuO, the Zeta potential of co-ground sample of PbO with sulfur also shows a positive movement about 16 mV, but remaining in more negative position than that of CuO, which might be one reason for the low efficiency of flotation.
3.5 Surface chemical compositions of various ground particles
In order to exactly understand the changes in particle surface chemical composition, the representative co-ground powder samples of CuO and PbO were subjected to EDS analyses and the related results are shown in Fig. 6.
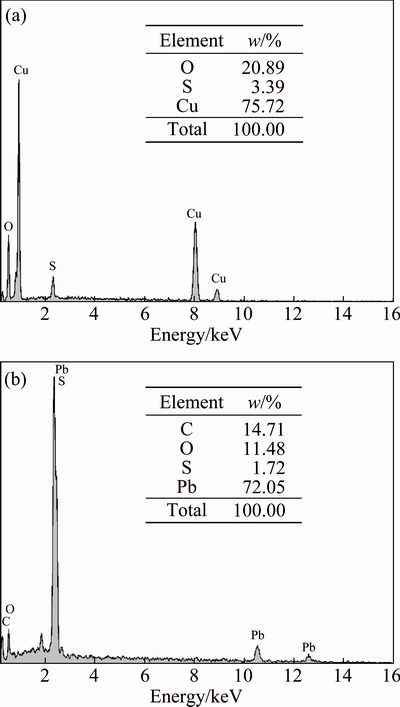
Fig. 6 EDS spectra of co-ground samples of CuO (a) and PbO (b)
These results show the uniform distribution of element sulfur on the surface of CuO particles and the formation of sulfide layer which is in favor of flotation. Although S is also detected in PbO sample, the occurrence of C with seven times content over S implies the overwhelming adhesion of carbon dioxide on particle surface. The contamination of CO2 on particle surface and the resulting surface carbonation may be a major reason of flotation deterioration.
The Raman spectra of CuO samples ground for 20 min with 5% sulfur at 200 and 500 r/min are shown in Fig. 7. From both samples, the peak in the range of 275-300 cm-1 attributed to Ag mode and the peak in 325-350 cm-1 attributed to Bg mode of CuO are observed, indicating with XRD analysis together that CuO phase has maintained the structure after grinding operation [33]. However, the peak intensity of the sample ground at 500 r/min is clearly weakened as a result of mechanical damage of crystal structure. No signal of CuS is detected from the sample ground at 200 r/min, while the peak positioned at 450-500 cm-1 from the sample ground at 500 r/min is attributed to Cu—S bond- stretching mode of Ag symmetric [34,35]. Different from the PbS appearance from PbO ground with S, no crystalline phase of copper sulfide can be detected by XRD analysis. The obvious Raman spectrum signal of Cu—S bonding implies that the combination between copper and sulfur might take place just on the surface and do not reach a formation of new sulfide phase.
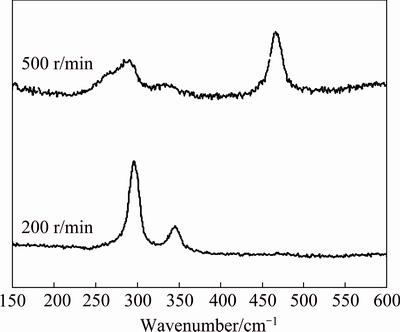
Fig. 7 Raman spectra of CuO samples ground for 20 min with 5% sulfur at 200 and 500 r/min
The EDS spectrum has already confirmed the abundance of carbon on the surface of the ground PbO particles. In order to further investigate the presence form of element carbon on particle surfaces, a FT-IR analysis of the PbO ground samples was carried out. Figure 8 shows the FT-IR spectra of PbO ground with sulfur at 500 r/min for 20 min and original sample without grinding, respectively. Compared with the original one, obvious changes are observed from the ground sample: appearances of OH- stretching vibration at about 3455 cm-1 and 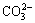 vibration mode positioned at around 1440 cm-1. It is understood that absorption of moisture and carbon dioxide on PbO sample during the grinding might proceed to give a composition similar to basic carbonate of lead.
vibration mode positioned at around 1440 cm-1. It is understood that absorption of moisture and carbon dioxide on PbO sample during the grinding might proceed to give a composition similar to basic carbonate of lead.
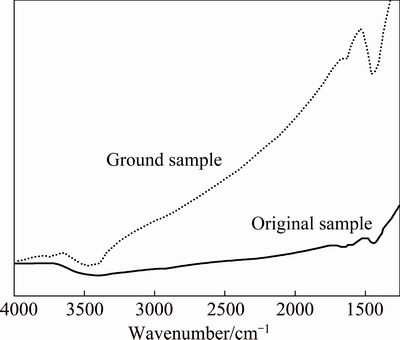
Fig. 8 FT-IR spectra of PbO samples ground for 20 min with 5% sulfur at 500 r/min and original sample without grinding
In addition to FT-IR analyses, DSC analysis was performed to investigate the surface carbonation of the ground PbO and the results are shown in Fig. 9. Compared with the original PbO, the sample ground at 500 r/min for 20 min gives a clear decomposition peak around 500 °C, suggesting the existence of lead carbonate [36].
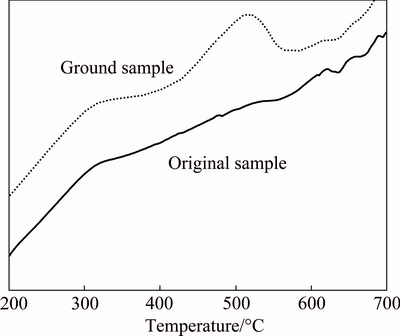
Fig. 9 DSC curves of original and ground samples of PbO
Above all, lead oxide shows high adsorption capacity to moisture and carbon dioxide after grinding, forming a carbonate-like surface. It can be confirmed that surface carbonation is the main factor deteriorating the flotation efficiency of PbO. Actually, several basic carbonates of Cu, Zn and Pb samples were tested by grinding with sulfur and no improvement in floatability was observed. Different approach is required to treat the carbonate-type minerals for raising floatability.
The changes in surface properties and floatability of the oxides reported here may serve as the basis to treat oxidized-type ores and solid wastes with the existences of non-ferrous metal oxides. The concept to control the surface modification by co-grinding with element sulfur to stay at chemical bonding from further to solid state reaction may help to develop some new processes to achieve the purpose to recover these nonferrous metals.
4 Conclusions
1) The floatability of CuO is improved after grinding with sulfur, by the formation of surface layer with properties similar to Cu sulfide due to the Cu—S bonding.
2) Deterioration in flotation efficiency of PbO after grinding operation is attributed to formation of several reaction products such as basic carbonate and PbS.
3) In the case of stable ZnO, no large change is observed with the compositions and the flotation recovery of the ground sample.
4) Our results may offer a new method to recover oxidized-type non-ferrous resources and provide a novel pathway to deal with various non-sulfidized metal materials.
References
[1] MEHDILO A, ZAREI H, IRANNAJAD M, ARJMANDFAR H. Flotation of zinc oxide ores by cationic and mixed collectors [J]. Minerals Engineering, 2012, 36-38: 331-334.
[2] HOSSEINI S H, FORSSBERG E. Physicochemical studies of smithsonite flotation using mixed anionic/cationic collector [J]. Minerals Engineering, 2007, 20: 621-624.
[3] ZHANG Qi-wu, WANG Jun, SAITO F, OKURA T, NAKAMURA I. Sulphidization of metal oxides by means of mechanochemical solid reaction [J]. Chemistry Letters, 2002, 11: 1094-1095.
[4] KE Yong, CHAI Li-yuan, MIN Xiao-bo, TANG Chong-jian, ZHOU Bo-sheng, CHEN Jie, YUAN Cui-yu. Behavior and effect of calcium during hydrothermal sulfidation and flotation of zinc-calcium-based neutralization sludge [J]. Minerals Engineering, 2015, 74: 68-78.
[5] CHAI Li-yuan, KE Yong, MIN Xiao-bo, ZHOU Bo-sheng, XUE Ke, CHEN Jie. Separation and recovery of ZnS from sulfidized neutralization sludge via the hydration conversion of CaSO4 into bulk CaSO4·2H2O crystals [J]. Separation and Purification Technology, 2015, 154: 76-81.
[6] KE Yong, MIN Xiao-bo, CHAI Li-yuan, ZHOU Bo-sheng, XUE Ke. Sulfidation behavior of Zn and ZnS crystal growth kinetics for Zn(OH)2-S-NaOH hydrothermal system [J]. Hydrometallurgy, 2016, 161: 166-173.
[7] MIN Xiao-bo, ZHOU Bo-sheng, KE Yong, CHAI Li-yuan, XUE Ke, ZHANG Chun, ZHAO Zong-wen, SHEN Chen. Sulfidation behavior of ZnFe2O4 roasted with pyrite: Sulfur inducing and sulfur-oxygen interface exchange mechanism [J]. Applied Surface Science, 2016, 371: 67-73.
[8] KE Yong, CHAI Li-yuan, LIANG Yan-jie, MIN Xiao-bo, YANG Zhi-hui, CHEN Jie, YUAN Sheng. Sulfidation of heavy-metal- containing metallurgical residue in wet-milling processing [J]. Minerals Engineering, 2013, 53: 136-143.
[9] KE Yong, CHAI Li-yuan, MIN Xiao-bo, TANG Chong-jian, CHEN Jie, WANG Yan, LIANG Yan-jie. Sulfidation of heavy-metal- containing neutralization sludge using zinc leaching residue as the sulfur source for metal recovery and stabilization [J]. Minerals Engineering, 2014, 61: 105-112.
[10] QIN Wen-qing, WANG Xing-jie, MA Li-yuan, JIAO Fen, LIU Rui-zeng, YANG Cong-ren, GAO Ke. Electrochemical characteristics and collectorless flotation behavior of galena: With and without the presence of pyrite [J]. Minerals Engineering, 2015, 74: 99-104.
[11] PEREZ-GARIBAY R, RAMIREZ-AGUILERA N, BOUCHARD J, RUBIO J. Froth flotation of sphalerite: Collector concentration, gas dispersion and particle size effects [J]. Minerals Engineering, 2014, 57: 72-78.
[12] OWUSU C, BRITO S, SKINNER W, ADDAI-MENSAH J, ZANIN M. The influence of pyrite content on the flotation of chalcopyrite/pyrite mixtures [J]. Minerals Engineering, 2014, 55: 87-95.
[13] CHEN Ai-liang, ZHAO Zhong-wei, JIA Xi-jun, LONG Shuang, HUO Guang-sheng, CHEN Xing-yu. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore [J]. Hydrometallurgy, 2009, 97: 228-232.
[14] LIU Qing, YANG Sheng-hai, CHEN Yong-ming, HE Jing, XUE Hao-tian. Selective recovery of lead from zinc oxide dust with alkaline Na2EDTA solution [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 1179-1186.
[15] ZHENG Yong-xing, LIU Wei, QIN Wen-qing, JIAO Fen, HAN Jun-wei, YANG Kang, LUO Hong-lin. Sulfidation roasting of lead and zinc carbonate with sulphur by temperature gradient method [J]. Journal of Central South University, 2015, 22: 1635-1642.
[16] SOLIHIN, ZHANG Qi-wu, TONGAMP W, SAITO F. Mechanochemical route for synthesizing KMgPO4 and NH4MgPO4 for application as slow-release fertilizers [J]. Industrial & Engineering Chemistry Research, 2010, 49: 2213-2216.
[17] FENG Qi-cheng, WEN Shu-ming, ZHAO Wen-juan, DENG Jiu-shuai, XIAN Yong-jun. Adsorption of sulfide ions on cerussite surfaces and implications for flotation [J]. Applied Surface Science, 2016, 360: 365-372.
[18] WU Dan-dan, WEN Shu-ming, DENG Jiu-shuai, LIU Jian, MAO Ying-bo. Study on the sulfidation behavior of smithsonite [J]. Applied Surface Science, 2015, 329: 315-320.
[19] FANG Wen-bin, LI Xue-wen, SUN Hong-fei, DING Yong-feng. Characterization of Ti-50%Al composite powder synthesized by high energy ball milling [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 333-337.
[20] ZHAO Zhong-wei, OUYANG K, WANG Ming. Structural macrokinetics of synthesizing ZnFe2O4 by mechanical ball milling [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1131-1135.
[21] CHEN Hui, MA Qin, SONG Qiu-xiang. Rapid synthesis of Mo5Si3-Al2O3 nanocomposite powders by mechanochemical reduction method [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1557-1562.
[22] KHAYATI G R, JANGHORBAN K. Preparation of nanostructure silver powders by mechanical decomposing and mechanochemical reduction of silver oxide [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 1520-1524.
[23] BALAZ P, ACHIMOVICOVA M. Mechano-chemical leaching in hydrometallurgy of complex sulphides [J]. Hydrometallurgy, 2006, 84: 60-68.
[24] BALAZ P, FICERIOVA J, LEONN C V. Silver leaching from a mechanochemically pretreated complex sulfide concentrate [J]. Hydrometallurgy, 2003, 70: 113-119.
[25] FICERIOVA J, BALAZ P, LEON C V. Thiosulfate leaching of silver, gold and bismuth from a complex sulfide concentrates [J]. Hydrometallurgy, 2005, 77: 35-39.
[26] BALAZ P, NGUYEN A V, FABIAN M, CHOLUJOVA D, PASTOREK M, SEDLAK J, BUJNAKOVA Z. Properties of arsenic sulphide As4S4 nanoparticles prepared by high-energy milling [J]. Powder Technology, 2011, 211: 232-236.
[27] BUJNAKOVA Z, DUTKOVA E, BALAZ M, TURIANICOVA E, BALAZ P. Stability studies of As4S4 nanosuspension prepared by wet milling in Poloxamer 407 [J]. International Journal of Pharmaceutics, 2015, 478: 187-192.
[28] WANG Jun, ZHANG Qi-wu, SAITO F. Improvement in the floatability of CuO by dry grinding with sulphur [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 302: 494-497.
[29] YUAN Wen-yi, LI Jin-hui, ZHANG Qi-wu, SAITO F. Mechanochemical sulfidization of lead oxides by grinding with sulfur [J]. Powder Technology, 2012, 230: 63-66.
[30] WANG Jun, LU Jin-feng, ZHANG Qi-wu, SAITO F. Mechanochemical sulfidization of nonferrous metal oxides by grinding with sulfur and iron [J]. Industrial & Engineering Chemistry Research, 2003, 42: 5813-5818.
[31] CHAI Li-yuan, LIANG Yan-jie, KE Yong, MIN Xiao-bo, TANG Cong-jian, ZHANG Hai-jing, XIE Xian-de, YUAN Cui-yu. Mechano-chemical sulfidization of zinc oxide by grinding with sulfur and reductive additives [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 1129-1138.
[32] BANZA A N, GOCK E. Mechanochemical processing of chrysocolla with sodium sulphide [J]. Minerals Engineering, 2003, 16: 1349-1354.
[33] MUKHERJEE N, SHOW B, MAJI S K, MADHU U, BHAR S K, MITRA B C, KHAN G G, MONDAL A. CuO nano-whiskers: Electrodeposition, Raman analysis, photoluminescence study and photocatalytic activity [J]. Materials Letters, 2011, 65: 3248-3250.
[34] BECUSSI M, BINI R, CASTELLUCCI E, ECKERT B, JODL H J. Mode assignment of sulfur α-S8 by polarized Raman and FTIR studies at low temperatures [J]. The Journal of Physical Chemistry B, 1997, 101: 2132-2137.
[35] MINCEVA-SUKAROVA B, NAJDOSKI M, GROZDANOV I, CHUNNILALL C J. Raman spectra of thin solid films of some metal sulfides [J]. Journal of Molecular Structure, 1997, 410-411: 267-270.
[36] REFAT M, ELSABAWY K. Infrared spectra, Raman laser, XRD, DSC/TGA and SEM investigations on the preparations of selenium metal, (Sb2O3, Ga2O3, SnO and HgO) oxides and lead carbonate with pure grade using acetamide precursors [J]. Bull Mater Sci, 2011, 34: 873-881.
硫磺共磨对铜铅锌氧化物表面性质和浮选的影响
李 钊,陈 敏,黄鹏武,张其武,宋少先
武汉理工大学 资源与环境工程学院,武汉 430070
摘 要:研究3种有色金属氧化物(CuO,PbO和ZnO)与单质硫璜在轻度干磨条件下的反应,并对磨后产物进行浮选实验,以期探索磨后产物的表面性质及可浮性的变化。考察磨后产物的相变、形貌特征、电化学性质及表面化学组成等。结果表明,通过Cu—S键合,在颗粒表面形成了具有类CuS性质的薄层,在与单质硫璜干磨后,CuO的可浮性明显提升。机械化学硫化处理后,PbO的可浮行下降,主要因为颗粒表面的碳酸盐化以及PbO与S之间发生生成PbS和PbSO4的歧化反应。机械化学硫化对ZnO的作用不大。
关键词:磨矿;有色金属氧化物;浮选;硫磺;回收
(Edited by Bing YANG)
Corresponding author: Qi-wu ZHANG; Tel: +86-17771490085; E-mail: zhangqw@what.edu.cn
DOI: 10.1016/S1003-6326(17)60274-7
Abstract: Co-grinding three nonferrous metal oxides (CuO, PbO and ZnO) with element sulphur under mild conditions and flotation of the ground samples were conducted to investigate the surface properties and floatability of the oxides. Phase transition, morphological features, electrochemical properties and surface chemical compositions of ground samples were studied. The results show that the floatability of CuO is improved after grinding with sulfur, by the formation of surface layer with properties similar to CuS due to the Cu—S bonding. The floatability of PbO is deteriorated after mechanochemical processing due to surface carbonation and the formation of PbS and PbSO4 by disproportionation reaction with sulfur. ZnO shows no evident response to mechanochemical sulfidation.


