
Effect of Nb on oxidation behavior of NiTiNb alloys
XU Jian(徐 舰), ZHAO Xin-qing(赵新青), GONG Sheng-kai(宫声凯)
School of Materials Science and Engineering, Beijing University of Aeronautics and Astronautics, Beijing 100083, China
Received 20 April 2006; accepted 30 June 2006
Abstract:
The oxidation behavior of NiTi and NiTiNb alloys containing different amounts of Nb (7%, 9%, mole fraction) were studied at 800 ℃ in air. It is found that the oxidation resistance of NiTi alloy can be effectively increased by the Nb addition. Under the same oxidation condition, the mass gain of NiTi is about 7 mg/cm2, while the mass gains are only 3 mg/cm2 for Ni47Ti44Nb9 alloy and 2.4 mg/cm2 for Ni52Ti41Nb7. Moreover the oxidation resistance of single phase NiTiNb alloy is better than that of the dual-phase alloy with large amount of Nb precipitates. On the basis of thermodynamics and kinetics of oxidation, the effect of Nb alloying element on the oxidation behavior of NiTi-based alloys was discussed.
Key words:
NiTi; NiTiNb; high-temperature oxidation; kinetics;
1 Introduction
NiTi based intermetallics are attractive functional materials because of their mechanical properties, good corrosion resistance, excellent biocompatibility, and shape memory effect as well as pseudoelasticity. As shape memory alloys, NiTi based alloys, including equiatomic NiTi, NiTiCu[1,2] and NiTiNb[3-5] have been found wide application in aerospace engineering, intelligent control and medical implant, etc. Recently, people have found that NiTi based intermetallics are also potential high temperature structural materials (NiTiAl alloys, for example). It is necessary to know about their oxidation behavior in atmospheric or high temperature environments. So far, only a few researches were reported on the oxidation behavior of the equiatomic NiTi intermetallics at elevated temperatures[6-8]. It has been recognized that the addition of Nb in aluminides, such as TiAl and NiAl, alters the oxidation kinetics and drastically improves their oxidation resistance at elevated temperatures[9-13]. The purpose of this work was to investigate the oxidation behavior of NiTi and NiTiNb alloys and the influence of Nb on the oxidation behavior of NiTi-based alloys at 800 ℃. The mechanism of the improvement of oxidation resistance of NiTi alloys by Nb addition was also discussed.
2 Experimental
2.1 Alloy preparation
Since the amount of Nb dissolved in NiTi matrix is very sensitive to Ni/Ti ratio in the alloy[14], different microstructures with single NiTi(Nb) phase and dual phase of NiTi(Nb) and b-Nb phase were achieved by a control of Ni/Ti ratio. The nominal compositions of the alloys were Ni50Ti50, Ni52Ti41Nb7 and Ni47Ti44Nb9. The high purity Ti (99.9% pure), Ni (99.9% pure) and Nb (99.9% pure) were used as the raw materials. All alloys, each about 40 g, were prepared by argon arc melting on a water-cooled copper hearth. All the ingots were homogenized at 850 ℃ for 24 h in vacuum and cooled down to room temperature in air. Specimens with dimensions of 5 mm×5 mm×10 mm were prepared by spark-cut, ground on emery papers and ultrasonically washed in acetone before oxidation.
2.2 Experimental procedures
The interrupted isothermal oxidation experiment was carried out at 800 ℃ in air. The specimens were laid obliquely in silicon dioxide crucibles to ensure that the six sides of the alloy had full contact with the air. Three specimens of each alloy were used for oxidation experiment in order to get the average mass change. The specimens were periodically removed from the furnace, air cooled, weighed, and returned to the furnace. The total oxidation time was 100 h. The mass gain was weighted by an electronic balance with accuracy of 0.1 mg. The microstructures of the specimen before and after the oxidation test were determined by D/max 2200pc X-ray diffraction. The surface morphology of the oxide scales and the cross section of the scales were observed and analyzed by scanning electron microscope (JSM5600) equipped with an X-ray energy-dispersive X-ray spectrometry (Link ISIS-300, Oxford Instru- ments).
3 Results
3.1 Microstructures
The back-scattered scanning electron microscope (SEM) micrographs (Fig.1) show that the microstructures of NiTi and Ni52Ti41Nb7 alloys are characterized by single phase, with no precipitates of β-Nb particles. By

Fig.1 Back-scattered SEM micrographs of alloys: (a) Ni50Ti50; (b) Ni52Ti41Nb7; (c) Ni47Ti44Nb9
contrast, the microstructure of Ni47Ti44Nb9 is composed of NiTi(Nb) matrix and large amount of eutectic phase of β-Nb, as shown in Fig.1(c). The energy-dispersive X-ray spectrometry analysis indicates that the average Nb contents of the matrix phase and β-Nb phase are, respectively, 4.86% and 79.2%(mole fraction).
3.2 Oxidation kinetics
The oxidation kinetics of the alloys performed at 800 ℃ is shown in Fig.2. The mass gain of NiTi alloy after cyclic oxidation test at 800 ℃ for 100 h is greater than 7 mg/cm2. The specimen with 7%Nb (mole fraction) performs the best oxidation resistance with a mass gain of 2.4 mg/cm2. It is worthy of noting that Ni52Ti41Nb7 alloy performs a better oxidation resistance than Ni47Ti44Nb9, though its Nb content is lower. The results suggest that the addition of Nb increases the oxidation resistance of NiTi alloys, especially when Nb forms solid solution in NiTi. After oxidation test at 800 ℃ for 100 h, only the NiTi alloys have oxide scale spallation found in the crucible. The other two alloys are covered by integrate light brown oxide scale.
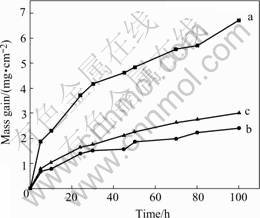
Fig.2 Kinetic curves of interrupted oxidation of NiTi and NiTiNb alloys at 800℃: (a) NiTi; (b) Ni52Ti41Nb7; (c) Ni47Ti44- Nb9
3.3 Scale morphology and composition
The microstructures and compositions of the oxide scales for different alloys were analyzed using X-ray diffraction and SEM/EDS. The surface morphologies of the three alloys after cyclic oxidation at 800 ℃ for 100 h are shown in Fig.3. The oxides on NiTi alloy are coarse polyhedral crystallites. With the addition of Nb, the oxide crystallites on the samples become finer and denser. It should be noted that the surfacial oxides on Ni47Ti44Nb9 alloy exhibit different feature from the other samples, revealing a rugged morphology, as shown in Fig.3(c). Considering the microstructure difference between Ni47Ti44Nb9 and the other alloys of single phase, it is speculated that the formation of the rugged oxide structure is associated with inhomogeneous distribution of Nb. The NiTi matrix containing a little Nb is apt to be oxidized relative to the high Nb areas (i.e. the eutectic of b-Nb and NiTi(Nb)), so that the oxidation rates are different from the low Nb areas to the high Nb areas, forming the rugged surfacial oxide morphology. The results of XRD and EDS show that the oxide scales for all the three alloys are composed of TiO2.
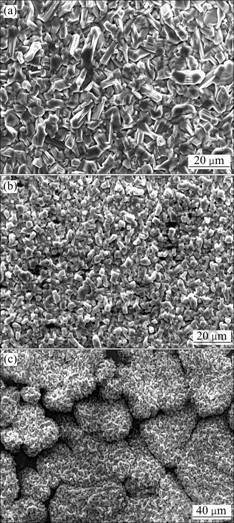
Fig.3 Surface SEM morphologies of alloy oxidized at 800 ℃ for 100 h: (a) NiTi; (b) Ni52Ti41Nb7; (c) Ni47Ti44Nb9
3.4 Cross sectional analysis
To study the formation characteristics of the oxide structures during high temperature oxidation of NiTi and NiTiNb alloys, the cross sectional morphologies of oxide scales as well as the substrate next to the oxide scales were observed by scanning electron microscopy. Meanwhile, the mapping of component elements, such as Ni, Ti, Nb, O were depicted to illustrate the distributions of these elements in the oxide scale and in the substrates, as shown in Figs.4-6.
From Fig.4, it can be seen that a multi-layer structure is formed on binary NiTi alloy. Combining the results of X-ray diffraction and SEM-EDS, the external scale is TiO2 about 20 μm in thickness, beneath it a NiTiO3 layer (average 6 μm in thickness) embeds in thick outer and inner TiO2 layers, showing a sandwich structure. The total thickness of the oxide scale is about 50 μm. Beneath the TiO2 layer, a Ti-depleted area of light-grey color, about 30 μm in thickness, is observed clearly. The analysis by energy-dispersive X-ray spectro- metry reveals that the stoichiometric composition of the Ti-depleted area is very close to Ni3Ti. This is similar to the results of previous investigations on binary NiTi alloys[6-8]. The cross-sectional morphology of Ni52Ti41Nb7 alloy is shown in Fig.5. The external scale is TiO2 layer about 20 μm in thickness, then a Nb-rich oxide layer exists beneath it, a Ni3Ti layer about 10 μm in thickness could also be observed adjoin to the substrate. The difference between Ni52Ti41Nb7 alloy and Ni50Ti50 alloy is no NiTiO3 layer formed, but having a Nb-rich layer between the TiO2 layer and Ni3Ti transition layer. From the thickness changes of the TiO2 and the transition layer, it can be seen that the main effect of Nb is to form a Nb-rich oxide layer beneath the external oxide scale and to prevent both the elements in the matrix and oxygen from diffusing through the TiO2 layer, so the speed of the oxidation is slowed down. As for Ni47Ti44Nb9 alloy, the matrix which is composed of equiatomic TiNi phase with little Nb solid solution, is easy to be oxidized, the thickness of the oxide scale is about 20 μm, while Nb precipitates are less oxidized and Nb in it tends to diffuse into the oxide layer.
4 Discussion
In the view of the oxidation thermodynamics, the Gibbs free energy for TiO2 formation is much lower than that for NiO. Accordingly, when oxidation occurs, the NiTi-based alloys are selectively oxidized and TiO2 forms preferentially on the surfaces, which is consistent with the results of X-ray diffraction. In general, the metallic oxide formation results from the mutual interdiffusion of metallic pieces and oxygen. From the kinetics point of view, the growth of TiO2 predominately depends on the diffusion of Ti ions, because of the chemical potential between the matrix and the subsurface underneath the oxide layer. With the outward diffusion of Ti, there leaves a Ti-depleted and Ni-rich layer beneath as-formed TiO2. For the equiatomic NiTi alloy, both Ti and oxygen interdiffuse through the TiO2 scale, and the Ni-rich layer can be oxidized to form complex oxide of Ni and Ti, i.e. TiNiO3. By comparison, the alloys with Nb addition have thinner TiO2 layer and no TiNiO3 is observed beneath the external TiO2 layer. In addition, a Nb-rich complex oxide layer is observed beneath TiO2 layer, as shown in Fig.5(a) and Fig.6(a). The Nb-rich
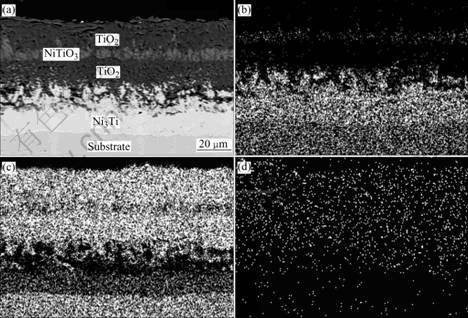
Fig.4 Cross sectional morphology of Ni50Ti50 oxidized 800 ℃ for 100 h (a) and mappings of Ni (b), Ti (c), O(d) elements
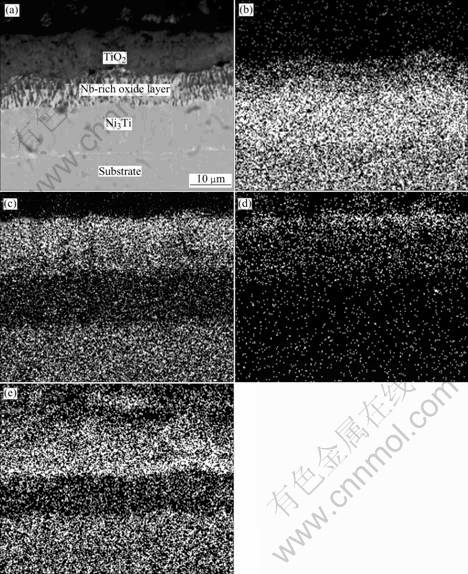
Fig.5 Cross sectional morphology of Ni52Ti41Nb7 oxidized 800 ℃ for 100 h (a) and mappings of Ni (b), Ti (c), O (d), Nb (e) elements

Fig.6 Cross sectional morphology of Ni47Ti44Nb9 oxidized of 800 ℃ for 100 h (a) and mappings of Ni (b), Ti (c), O(d), Nb (e) elements
oxide layer can effectively prevent oxygen from inward diffusion, inhibiting the formation of TiNiO3. Wagner’s theory pointed out that the growth of TiO2 layer depends on Ti ions diffusing outwardly. According to the present experimental results, the thin TiO2 layer formation indicates that this Nb-rich oxide layer impedes outward diffusion of Ti ions in the matrix. There are some differences for Ni47Ti44Nb9 alloy under the same oxidation condition. First, the thickness of TiO2 on this alloy is thicker than that on Ni52Ti41Nb7, though it has larger amount of Nb. From the cross section morphology of oxide scale and the mapping of Nb element, it can be seen that there exists Nb-rich oxides in TiO2 layer. It is reasonably speculated that the Nb-rich oxides results from the outward diffusing of Nb precipitates. It shows that though the content of Nb in Ni47Ti44Nb9 is more than that in Ni52Ti41Nb7, some of Nb will form isolated Nb precipitates, which tend to enter into the TiO2 layer after oxidation, but not form Nb-rich oxide layer underneath the oxide scale. The forming of Nb-rich oxide layer beneath TiO2 depends on the Nb solid solution in the NiTi matrix. This means that Nb can effectively improve the oxidation of NiTi-based alloys especially when it forms the Nb solid solution.
For TiNiNb alloys with single phase, the Nb disperses evenly in the alloy and contributes to form thick Nb-rich oxide layer beneath the TiO2 layer, which can effectively prevent the oxygen and metallic ions in substrate diffusing through it. The oxidation resistance is improved. For the alloys with two phases, a large amount of Nb precipitates are easy to enter into the external TiO2 layer not to form Nb-rich sub-surface layer. It can be seen that the efficient way to improve the anti-oxidation ability of the alloy is to gain the single phase with the largest amount of Nb solid solution.
5 Conclusions
On the basis of the above investigation on the oxidation behavior of the Ni50Ti50 and NiTiNb with Nb (7%,9%), especially the beneficial effect of Nb on the oxidation resistance of the NiTi alloy, the following conclusions are obtained.
1) The Ni52Ti41Nb7 alloy with single phase has the best oxidation resistance among the three alloys. The mass gain after 100 h cyclic oxidation at 800 ℃ is 2.4 mg/cm2, while that of the NiTi is about 7 mg/cm2.
2) The NiTi alloys are oxidized seriously, from the surface to the matrix the scales are TiO2, NiTiO3, TiO2 and transition Ni3Ti layer.
3) Nb has the ability to improve the oxidation resistance of NiTi alloys. It can form a Nb-rich layer beneath the external oxide scale, which can effectively impede the diffusion of oxygen ion and the elements in the matrix.
4) The oxidation resistance of the single phase NiTiNb alloys with much amount of Nb solid solution is much better than that of the dual phase alloys with large amount of Nb precipitates.
References
[1] DANILOV A, KAPANEN A. Biocompatibility of austenite and martensite phases in NiTi-based alloys [J]. Journal De Physique IV, 2003, 112:1117-1120.
[2] GIL F J, PLANELL J A. Effect of copper addition on the superelastic behavior of Ni-Ti shape memory alloys for orthodontic applications [J]. Journal of Biomedical Materials Research, 1999, 48: 682-688.
[3] OTSUKA K, WAYMAN C M. Shape Memory Materials [M]. London: Cambridge University Press, 1998. 225-230.
[4] DUERIG T W, MELTON K, PROFT J L. Engineering Aspects of Shape Memory Alloys [M]. London: Butterworth-Heinemann, 1990. 130.
[5] PIAO M, MIYAZAKI S, OTSUKA K. Characteristics of deformation and transformation in Ti44Ni47Nb9 memory alloy [J]. Mater Trans JIM, 1992, 33: 341-351.
[6] XU C H, MA X Q, SHI S Q, et al. Oxidation behavior of TiNi shape memory alloy at 450-750 ℃ [J]. Mater Sci Eng A, 2004, 371: 45-50.
[7] FIRSTOV G S, VITCHEV R G., KUMAR H, BLANPAIN B, VAN HUMBEECK J. Surface oxidation of NiTi shape memory alloy [J]. Biomaterials, 2002, 23: 4863-4871.
[8] CHU C L, WU S K, YEN Y C. Oxidation behavior of equiatomic TiNi alloy in high temperature air environment [J]. Mater Sci Eng A, 1996, 216: 193-200.
[9] ROY T K, BALASUBRAMANIAM R, GHOSH A. High- temperature oxidation of Ti3Al-based titanium aluminides in oxygen [J]. Metall Mater Trans, 1996, 27A: 3993-4002.
[10] VARMA S K, CHAN A, MAHAPATRA B N. Static and cyclic oxidation of Ti-44Al and Ti-44Al-xNb alloys [J]. Oxid Met, 2001, 55: 423-435.
[11] SHIDA Y, ANADA H. The effect of various ternary additives on the oxidation behavior of TiAl in high-temperature air [J]. Oxid Met, 1996, 45: 197-219.
[12] YOSHIHARA M, MIURA K. Effects of Nb addition on oxidation behavior of TiAl [J]. Intermetallics, 1995, 3: 357-363.
[13] PEREZ P, HAANAPPEL V A C, STROOSNIJDER M F. Effect of niobium on the oxidation behaviour of titanium in N2/20%O2 atmospheres [J]. Mater Sci Eng A, 2000, 284: 126.
[14] YANG Y Z, ZHAO X Q, MENG L J. Microstructure and transformation behavior of Ni-Ti-Nb shape alloys with low Nb content [J]. Acta Metallurgica Sinica, 2005, 41(6): 627-632. (in Chinese)
Corresponding author: GONG Sheng-kai; Tel: +86-10-82317117; E-mail: gongsk@buaa.edu.cn


