J. Cent. South Univ. (2016) 23: 2492-2499
DOI: 10.1007/s11771-016-3308-5
Microstructural evolution of GCr15 steel during austenitizing and quenching considering C and Cr content
LIU Qing-long(刘青龙)1, 3, QIAN Dong-sheng(钱东升)1, 3, WEI Wen-ting(魏文婷)2, 3
1. School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China;
2. School of Automotive Engineering, Wuhan University of Technology, Wuhan 430070, China;
3. Hubei Key Laboratory of Advanced Technology for Automotive Components, Wuhan 430070, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
Microstructural evolution of GCr15 steels with different C and Cr contents during austenitizing and quenching was studied. Thermodynamic analysis of cementite dissolution was implied to obtain the critical temperature. The coordination number x in FexCr3-xC and the volume fraction of undissolved cementite were computed according to element conservation and equilibrium phase diagram. The MS (martensite transformation temperature) was calculated by using empirical formula. The retained austenite content was calculated with further consideration of quenching temperature. The results showed that the coordination number and the undissolved cementite content were promoted by the austenitizing temperature and carbon content of the steel. Increasing Cr element reduced the coordination number.GCr15 steels with different components had nearly the same MS when austenitization at 830 °C to 860 °C. The interaction of C and Cr complicated the evolution of MS and retained austenite content. The results were in good agreement with the literature, which could guide to obtain specified retained austenite and/or carbides.
Key words:
GCr15 steel; austenitization; quenching; thermodynamic calculation; kinetics;
1 Introduction
To obtain high wear resistance and good fatigue properties has been a subject of intense and long-lasting research in bearing industry [1]. As a widely used high-carbon steel, GCr15 provides high strength, high wear resistance and good fatigue properties when it`s transformed into either a martensitic and/or bainitic state [2]. Hence, quenching process maintains the main approach to obtain proper strength-toughness combinations [3].
Generally, the martensitic quenching process of GCr15 consists of austenitization and martensite transformation [4]. The austenitization can be divided into two stages. The first stage is the transformation of ferrite to austenite, followed by the hypereutectoid carbide dissolution into the matrix in the second stage [5]. Then the components are cooled at a rapid rate to produce martensite.
Considerable researches were involved with the GCr15 bearing steel quenching process. BESWICK [6] discussed the effect of chromium in GCr15 steel. The ferrite-to- austenite transformation temperature increased with the Cr content and the dissolution rate of spheroidized carbides was enhanced by the rate of chromium diffusion from the carbide-matrix interface. MA et al [7] and DONG [8] studied the influence of quenching medium temperature on microstructure and mechanical properties respectively. Their researches pointed out that the amount of retained austenite was hardly changed when quenching temperature was 40 °C and 60 °C in oil, but increased markedly over 80 °C. MASON and  [9] applied three iterations designed by Taguchi experiments to determine the optimal quenching treatments for the purposes of minimizing retained austenite content and maximizing Rockwell hardness. The experiments indicated that the austenitizing temperature brought great impact on retained austenite content and hardness. Numerical simulations focusing on the phase content and residual stresses also accounted for the quenching process [10-12].
[9] applied three iterations designed by Taguchi experiments to determine the optimal quenching treatments for the purposes of minimizing retained austenite content and maximizing Rockwell hardness. The experiments indicated that the austenitizing temperature brought great impact on retained austenite content and hardness. Numerical simulations focusing on the phase content and residual stresses also accounted for the quenching process [10-12].
Some recent studies expounded the following findings. The carbon content in quenched martensite had appreciable impact on the tempering strain, and therefore affected the dimensional stability of component [13]. The thermal stability of retained austenite increased along with the carbon content [14], while the stability of transition carbide was influenced by the joint effect of carbon and alloying element contents, especially Cr [15-16]. Those results suggested the necessity of conducting more thorough and precise researches to understand the relations between microstructure and properties. Noteworthy, the element contents in GCr15 steel manufactured according to different national standards vary notably, especially the C and Cr contents. Fluctuation of elements contents in the same batch of steel is also common. However, unchanged quenching process parameters will bring undesirable microstructure and reduce the components’ reliability. Therefore, it is quite necessary to establish the microstructural evolution model of GCr15 during quenching process with the subtle compositions differences and elements partition into consideration.
In this work, a method was proposed to characterize the microstructural evolution of GCr15 during austenitizing and quenching. The method was based on the principle that carbon content in austenitic matrix during austenitization was determined by the dissolution of carbides, which should comply with the solid solubility formula at a given temperature. The volume fraction of retained austenite was calculated using an empirical formula, and the content of undissolved carbide was predicted according to the law of conservation of carbon element. The computation was obtained by iterative solution method with MATLAB and was validated with the experimental measurements in literature.
2 Simulation model
In order to analyze the microstructural evolution and elements partitioning behavior, the following hypotheses were made: 1) The oxidation and decarburization was negligible; 2) The cooling capacity of quenching medium was constant; 3) The effects of austenitizing heating speed were ignorable; 4) The carbide was thought to be cementite ((Fe, Cr)3C) throughout the whole process. The first three hypotheses were similar to the actual situation with the use of advanced heat treatment equipment and forced convection devices in manufacturing. As for the last one, the fact that a series of alloy carbides such as (Fe, Cr)3C, (Fe, Cr)7C3, (Fe, Cr)23C7 existed was ignored. This assumption was intended to reduce computation load.
2.1 Austenitization stage
The study on the austenitization of GCr15 bearing steel was concentrated in the two-phase region, meaning that the ferrite-to-austenite transformation was already finished and the dissolution of cementite into austenite was concerned in this stage.
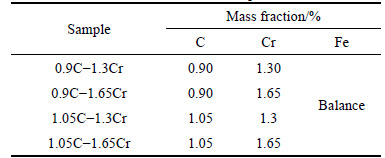
An overall consideration of all the alloy elements would cause huge computational burden. Hence, the effects of C and Cr content were taken into account and Fe was set as balance. The carbon content was ranged from 0.9% to 1.05% and 1.30% to 1.65% in mass fraction for chromium according to the upper and lower bounds of the national standards of GCr15. Four steels with different carbon and chromium contents were selected as special cases, as listed in Table 1. The abbreviation of 0.9C-1.3Cr meant that the chemical composition of bearing steel was of 0.9% carbon, 1.3% chromium and Fe for balance. The lower limit of austenitizing temperature was 750 °C.
Table 1 Compositions of GCr15 steels used to calculate volume fraction and element contents of phases
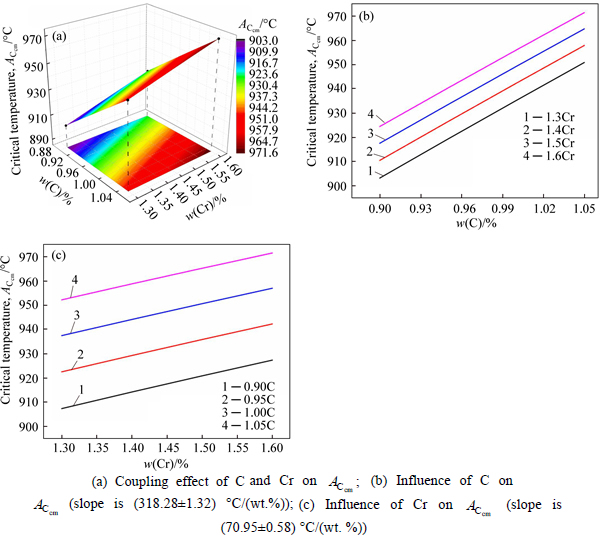
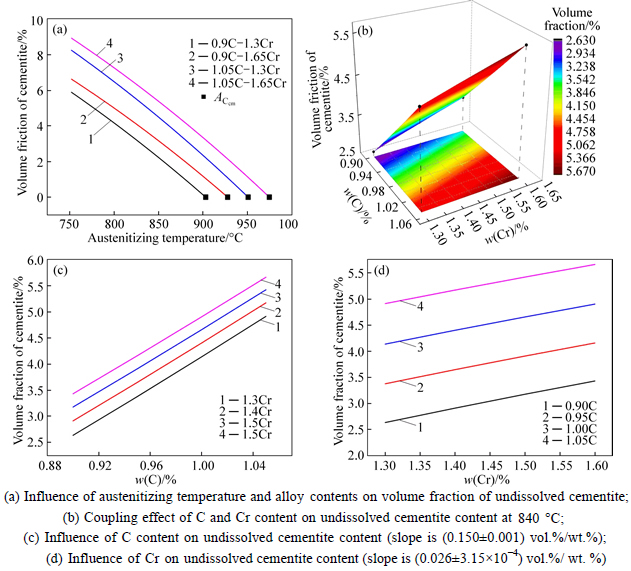
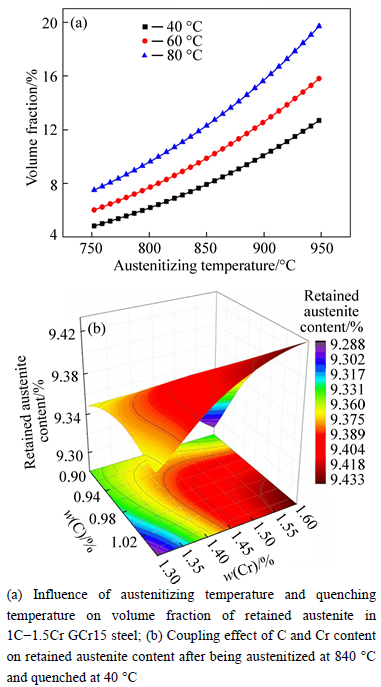
The microstructure could be considered to reach transient equilibrium at each temperature increment when minimizing the discrete time steps during heating. Hence, the cementite had a particular chemical formula to a steel with certain compositions and can be rewritten as FexCr3-xC where x is the coordination number (0 The thermodynamic definition of the equilibrium constant was as follows: where [Fe], [Cr] and [C] were the equilibrium solubility of iron, chromium and carbon elements in austenite, respectively; [FexCr3-xC] was concentration. The chemical reaction could be decomposed as follows: Hence, the corresponding reaction equilibrium constant could be driven to where The elements in the austenitic matrix must comply with the austenitizing temperature formula, and the proportions of Fe, Cr and C in cementite should be compatible with the stoichiometric FexCr3-xC: where A1, B1, A2 and B2 were constants in the solid solubility formula of Fe3C and Cr3C, respectively; MFe, MCr and MC stood for the relative atomic mass of iron, chromium and carbon, respectively; w(Fe), w(Cr) and w(C) meant the mass fractions of iron, chromium and carbon in GCr15 steel, respectively. The algorithm of iteration was implemented in MATLAB, and detailed calculation process could refer to Ref. [17]. The equilibrium solubility of iron, chromium and carbon in austenite and the coordination number x in FexCr3-xC could be confirmed at an established temperature T by solving the simultaneous Eqs. (9) to (12). Therefore, the mass fraction of chromium and carbon in cementite could be written as w(Cr)-[Cr] and w(C)-[C], respectively. The volume fraction of undissolved cementite that equilibrium precipitated at certain temperature T can be obtained where ργ and ρcementite are the densities of austenite and cementite, respectively. When the equilibrium solubilities of chromium and carbon in austenite equal their corresponding element contents of the GCr15 steel, the cementite dissolved into the austenite completely. The corresponding temperature could be calculated as where 2.2 Quenching stage According to the calculated results of austenitizing stage and the compositions of selected steels, the martensitic transformation temperature and the influence of austenitizing processes were computed. An empirical formula considering alloy elements interaction was used to calculate the martensitic transformation temperature, as follows [18]: where Ms was the martensitic transformation temperature; w(C), w(Ni), w(Cr), w(Mo) and w(Mn) represent the mass fractions of carbon, nickel, chromium, molybdenum and manganese in GCr15, respectively. The reliability of empirical formula has been confirmed by the central atom model [18]. The volume fraction of retained austenite could be calculated as where Vγ was the volume fraction of retained austenite and Tq was the quenching medium temperature ranging from 40 °C to 80 °C. 3 Results and discussion Slight differences among the alloy compositions lead to a significant diversity of the critical temperatures, as shown in Fig. 1(a). With rising carbon and chromium content, the temperature at which carbides dissolve completely increases gradually. The variance between the temperatures of 1.05C-1.65Cr and 0.9C-1.3Cr is up to 71.6 °C. Noteworthy, the C content promotes the critical temperature more effectively. An incensement in carbon content by 0.1% increases the value by about 31.9 °C while the increment is nearly 7.1 °C for 0.1% more chromium, as fitted in Figs. 1(b) and (c). Consequently, the GCr15 steel with lower C and Cr content (i.e. sample 0.9C-1.3Cr) will present less carbide and coarse grain accompanied by poor mechanical properties after quenching if the steels with different compositions are implemented to the same austenitizing process without distinction. The impacts of austenitizing temperature and the alloy compositions of steel on the coordination number x in FexCr3-xC are shown in Fig. 2. The coordination number is generally determined by the temperature and concentration gradient at the cementite/austenite interface, and the later one is influenced by the alloy content. In general, to the steel with certain alloy compositions, the coordination number decreases with the austenitizing temperature, as shown in Fig. 2(a). The overlap of the coordination number in 0.9C-1.3Cr and 1.05C-1.6Cr implies that the affecting rules of the contents of C, Cr are different from each other. To steels with different compositions at the same austenitizing temperature 840℃, the coordination number rises along with the increase of the carbon content and decrease of the chromium content, as shown in Fig. 2(b). An augmentation in the carbon content by 0.1% increased the coordination number value by about 0.042 while the increment was nearly 0.023% for 0.1% less chromium, as fitted in Figs. 2(c) and (d). Fig. 1 Influence of element composition on Fig. 2 Influence of element content on coordination number of cementite in GCr15: The strength and wear resistance of secondary carbide phase are decided by its type and the coordination number, which varies with austenitizing temperature and alloy compositions. The distortion of crystal lattice of cementite is engendered when the Fe atoms are substituted by Cr. The cementite lattice distortion increases along with the coordination number, which brings the carbides better strength and wear resistance. The calculation results in Fig. 3 reveal the effect of the contents of C, Cr and austenitizing temperature on undissolved cementite. The carbides undissolved during austenization stage retain after quenching, providing the steel high wear resistance and mechanical properties. The results in Fig. 3(a) indicate that there is an exponential relationship between volume fraction and austenitizing temperature to the steel with certain compositions. The volume fraction of undissolved cementite rises along with the increase of the carbon and chromium content, as shown in Fig. 3(b). The difference of cementite content between 1.05C-1.65Cr steel and 0.9C-1.3Cr steel is up to 0.12% at 840 °C. Although the carbon content strongly promotes the volume fraction of cementite, as shown in Fig. 3(c), carbon element distributes in the matrix evenly due to its high diffusion coefficient at austenitizing temperature. Meanwhile, chromium with relatively low diffusion coefficient becomes the main factors influencing the local volume fraction of carbides, as presented in Fig. 3(d). Hence, reducing the banded structure of bearing materials is an important premise to obtain a homogeneous microstructure. Fig. 3 Influence of element content on undissolved cementite content in GCr15: The calculation results of the martensitic transformation temperature MS are presented in Fig. 4. To a certain GCr15, the Ms is linearly correlated with austenitizing temperature. Within the temperature range from 830 °C to 860 °C, GCr15 steels with different components present similar martensitic transformation temperature. Therefore, the influence of austenitizing temperature should be considered. The element contents in the austenite matrix and that in the steel are quite different at conventional austenitizing temperature because a number of carbides remain undissolved. A more visualized result of GCr15 steels austenitized at 840 °C was presented in Fig. 4(b). The projection of the variation tendency of Ms versus C and Cr content demonstrated C-curve relationship. Increasing carbon and chromium content reduced the Ms. And the effect of carbon is more significant when the Cr content is higher. The abnormal computed result of 1.05C-1.3Cr suggests that the interaction of carbon and chromium is rather prominent. The effects of austenitizing temperature and quenching temperature on the volume fraction of retained austenite in 1C-1.5Cr GCr15 steel are represented in Fig. 5(a). When the austenitizing temperature is constant, higher quenching temperature leads to more retained austenite, which is good agreement with previous findings [7-8]. The volume fraction of retained austenite increases along with the austenitizing temperature at a certain quenching temperature. Obviously, the influence of the quenching temperature is more significant. These rules are applicative in other GCr15 steel with different alloy contents. Fig. 4 Evolution of martensite transformation temperature during quenching: The computational retained austenite contents of GCr15 with different alloy contents after being austenitized at 840 °C and quenched at 40 °C are shown in Fig. 5(b). The projection of the relationship between volume fractions of retained austenite versus alloy contents demonstrates a similar C-curve like Ms. However, the volume fraction of retained austenite increases along with carbon and chromium content and the effect of carbon seems to be more significant when the Cr content is higher. The interactions of alloy elements make the relationship between the retained austenite content and alloy contents strongly nonlinear. Fig. 5 Evolution of retained austenite after quenching: Based on the kinetics of phase transformation during quenching, the retained austenite content is determined by the undercooling between quenching temperature and martensite transformation temperature. When the quenching temperature is constant, the austenite temperature affectes the element contents of the matrix, and then affects the MS. The element contents of the matrix have little difference. Therefore, the retained austenite contents are almost the same. The calculation results of retained austenite well coincide with those experimental data in the current researches [19-21], as shown in Fig. 6. The quenching temperature of 0.9C-1.34Cr is 50 °C and that of 1.05-1.6Cr is 70 °C in the calculation. Fig. 6 Comparison between construction results and literature data In field experience, the metastable iron-cementite equilibrium is more common during austenitizing before quenching. The uneven element distribution in the matrix promotes the regional distribution of microstructure, especially retained austenite. Estimating the volume fraction of retained austenite of GCr15 steel may help to understand dimensional change during tempering and then the dimensional stability of the components. 4 Conclusions Dissolution kinetics of cementite was calculated to obtain the critical temperature, and then the coordination number x and equilibrium content of cementite were computed according to element conservation. The martensite transformation temperature Ms was calculated by using empirical formula and the volume fraction of retained austenite was calculated as well. The simulation has been proved with experimental results. Some rules have been revealed: 1) The Cr element promotes the critical temperature more effectively. Increasing the Cr content would postpone the dissolution of cementite. 2) The coordination number x in FexCr3-xC increases along with the C and Cr content, but the variation range is small. 3) Undissolved cementite content increases along with the C and Cr content, and the prior one is more important. 4) The evolution of Ms is determined by the content of alloy elements in the matrix. The overlap of Ms during 830 °C to 860 °C indicates the interaction of C and Cr elements. 5) The evolution of retained austenite fraction is complex and the effect of austenitizing and quenching temperature is dominant. Acknowledgment The authors would like to thank a grant from the Marie Curie Actions-IRSES (PIRSES-GA-2012-318968), and the Fundamental Research Funds for the Central Universities ,China(WUT: 2016IVA042). References [1] CHAKRABORTY J, CHATTOPADHYAY P P, BHATTACHARJEE D, MANNA I. Microstructural refinement of bainite and martensite for enhanced strength and toughness in high-carbon low-alloy steel [J]. Metallurgical & Materials Transactions A, 2010, 41(11): 2871-2879. [2] BARROW A T W, KANG J H, [3] BHADESHIA H K D H. Steels for bearings [J]. Progress in Materials Science, 2012, 57(2): 268-435. [4] MIRANDA R. Phase transformations in steels, Volume 1: Fundamentals and diffusion-controlled transformations [J]. International Journal of Environmental Studies, 2013, 70(2): 337-338. [5] SONG W, CHOI P P, INDEN G, PRAHL U, RAABE D, BLECK W. On the spheroidized carbide dissolution and elemental partitioning in high carbon bearing steel 100Cr6 [J]. Metallurgical & Materials Transactions A, 2014, 45(2): 595-606. [6] BESWICK J M. The effect of chromium in high carbon bearing steels [J]. Metallurgical & Materials Transactions A, 1987, 18(11): 1897-1906. [7] MA Qin, LU Xue-mei, ZHANG Wen. Influence of quenching medium temperature on microstructure and mechanical properties of GCr15 steel [J]. Heat Treatment of Metals, 2011, 36(1): 80-83. [8] DONG Xiao-ping. Duplex quenched structure of steel GCr15 and its influence on mechanical properties [J]. Bearing, 2013(8): 33-38. [9] MASON P W, [10] ZHOU Zhi-fang, WANG Xiao-yan, GU Jian-feng. Numerical simulation of eccentric cylinder quenching process [J]. Journal of Mechanical Engineering, 2011, 47(12): 62-63. [11] YAO Xin, GU Jian-feng, HU Ming-juan, ZHANG Wei. Numerical simulation of the quenching process of GCr15 steel tube [J]. Transactions of Metal Heat Treatment, 2003, 24(1): 78-81. [12] ZHU Li. Simulation and analysis of quenching process for hollow cylindrical steel casting [J]. Foundry Technology, 2014, 35(1): 63-65. [13] PEREZ M, SIDOROFF C, VINCENT A, ESNOUF C. Microstructural evolution of martensitic 100Cr6 bearing steel during tempering: From thermoelectric power measurements to the prediction of dimensional changes [J]. Acta Materialia, 2009, 57(11): 3170-3181. [14] LUZGINOVA N, ZHAO L, SIETSMA J. Evolution and thermal stability of retained austenite in SAE 52100 bainitic steel [J]. Materials Science & Engineering A, 2007, 448(s1/2): 104-110. [15] PRECIADO M, PELLIZZARI M. Influence of deep cryogenic treatment on the thermal decomposition of Fe–C martensite [J]. Journal of Materials Science, 2014, 49(23): 8183-8191. [16] MORRA P V, [17] YONG Qi-long. The second phases in steel and iron [M]. Beijing: Metallurgical Industry Press, 2006. (in Chinese) [18] XU Zu-yao. Martensitic transformation and martensite [M]. Beijing: Science Press, 1980. (in Chinese) [19] WENG Wei-shan. Experimental studies on the heat treatment process and performance of GCr4 and GCr15 steels [J]. Bearing, 1986(2): 23-28. (in Chinese) [20] YE Jian-yi, GAO Yuan-an, XIE Qian. Comparing analysis on impact toughness and contact fatigue life of GCr4 and GCr15 steels [J]. Bearing, 2003(2): 30-32. (in Chinese) [21] LI Chao, WANG Jian-li. Research on bainite + martensite duplex microstructure and its mechanical properties in SAE 52100 bearing steel [J]. Iron and Steel, 1989, 24(5): 45-49. (in Chinese) (Edited by YANG Hua) Foundation item: Project(51575414) supported by National Natural Science Foundation of China; Project(IRT13087) supported by the Innovative Research Team Development Program of Ministry of Education of China; Project(2015AAA005) supported by the project of Important Science and Technology Innovation Program of Hubei Province, China Received date: 2016-04-12; Accepted date: 2016-08-20 Corresponding author: QIAN Dong-sheng, PhD; Tel: +86-27-87168391; E-mail: qiands@whut.edu.cn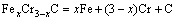 (1)
(1)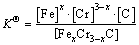 (2)
(2)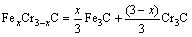 (3)
(3)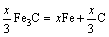 (4)
(4)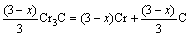 (5)
(5)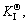
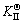 and
and 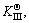 as listed below:
as listed below: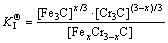 (6)
(6) was actually the hybrid entropy composed by Fe3C and Cr3C dissolved in each other. In another words, the effective activities of Fe3C and Cr3C in FexCr3-xC was (x/3) and ((3-x)/3), respectively. Solid solubility formula of binary phase could still be used to calculate the activity of Fe3C and Cr3C, leading to the following:
was actually the hybrid entropy composed by Fe3C and Cr3C dissolved in each other. In another words, the effective activities of Fe3C and Cr3C in FexCr3-xC was (x/3) and ((3-x)/3), respectively. Solid solubility formula of binary phase could still be used to calculate the activity of Fe3C and Cr3C, leading to the following: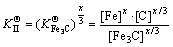 (7)
(7)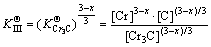 (8)
(8)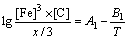 (9)
(9)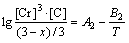 (10)
(10)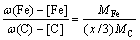 (11)
(11)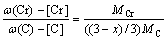 (12)
(12)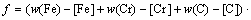
 (13)
(13)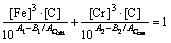 (14)
(14)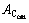 was the temperature at which all carbides dissolved into austenite.
was the temperature at which all carbides dissolved into austenite.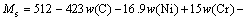
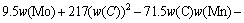
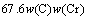 (15)
(15)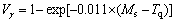 (16)
(16)
 of GCr15:
of GCr15: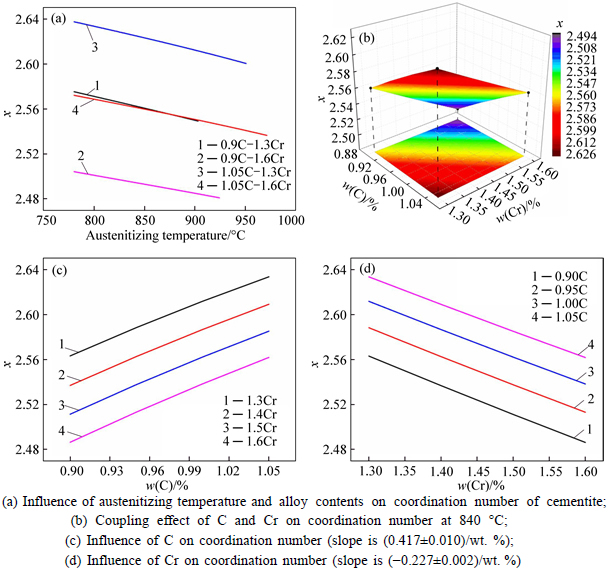


 -DEL-CASTILLO P E J. The ∈→η→θ transition in 100Cr6 and its effect on mechanical properties [J]. Acta Materialia, 2012, 60(s6/7): 2805-2815.
-DEL-CASTILLO P E J. The ∈→η→θ transition in 100Cr6 and its effect on mechanical properties [J]. Acta Materialia, 2012, 60(s6/7): 2805-2815. P S. Iterative taguchi analysis: Optimizing the austenite content and hardness in 52100 steel [J]. Journal of Materials Engineering & Performance, 2001, 10(1): 14-21.
P S. Iterative taguchi analysis: Optimizing the austenite content and hardness in 52100 steel [J]. Journal of Materials Engineering & Performance, 2001, 10(1): 14-21. A J, MITTEMEIJER E J. Decomposition of iron-based martensite: A kinetic analysis by means of differential scanning calorimetry and dilatometry [J]. Journal of Thermal Analysis & Calorimetry, 2001, 64(3): 905-914(10).
A J, MITTEMEIJER E J. Decomposition of iron-based martensite: A kinetic analysis by means of differential scanning calorimetry and dilatometry [J]. Journal of Thermal Analysis & Calorimetry, 2001, 64(3): 905-914(10).
Abstract: Microstructural evolution of GCr15 steels with different C and Cr contents during austenitizing and quenching was studied. Thermodynamic analysis of cementite dissolution was implied to obtain the critical temperature. The coordination number x in FexCr3-xC and the volume fraction of undissolved cementite were computed according to element conservation and equilibrium phase diagram. The MS (martensite transformation temperature) was calculated by using empirical formula. The retained austenite content was calculated with further consideration of quenching temperature. The results showed that the coordination number and the undissolved cementite content were promoted by the austenitizing temperature and carbon content of the steel. Increasing Cr element reduced the coordination number.GCr15 steels with different components had nearly the same MS when austenitization at 830 °C to 860 °C. The interaction of C and Cr complicated the evolution of MS and retained austenite content. The results were in good agreement with the literature, which could guide to obtain specified retained austenite and/or carbides.


