
Bioleaching of chalcopyrite with Acidianus manzaensis YN25 under contact and non-contact conditions
ZHANG Li-min(张立民)1, PENG Juan-hua(彭娟花)1, WEI Man-man(魏曼曼)1,
DING Jian-nan(丁建南)2, ZHOU Hong-bo(周洪波)1, 2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy, Ministry of Education, Central South University, Changsha 410083, China
Received 25 August 2009; accepted 6 January 2010
Abstract:
In order to investigate the contributions of contact and non-contact cells of Acidianus manzaensis (A. manzaensis) YN25 to the bioleaching of chalcopyrite, three experiments were carried out in the modified shake flasks. The redox potential, pH, cell density, copper and iron ions in the solution were monitored, and the morphological feature and chemical composition of the leached residues were analyzed. The highest leaching efficiency of Cu and Fe was reached in the experiment where the A.manzaensis YN25 could contact the surface of the chalcopyrite. There was no precipitation of jarosite in the leached residues of three experiments, but there was elemental sulfur in the leached residues when the cells could not contact the chalcopyrite. From these results, it is apparent that the leaching of the chalcopyrite is the cooperative action of the contact and non-contact A. manzaensis YN25.
Key words:
A. manzaensis YN25; bioleaching; chalcopyrite; elemental sulfur;
1 Introduction
Chalcopyrite is both the most abundant and the most refractory copper sulfide. Several studies with mesophilic microorganisms such as Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans have shown very slow copper leaching rates[1]. The reason that chalcopyrite does not respond well to mesophiles is primarily its tendency to “passivate” at the high solution potentials (>600 mV vs Ag/AgCl). However, when thermophilic microorganisms were used, leaching rates were considerably enhanced due to high temperatures and the metabolic characteristics of these types of microorganisms[2-3]. The researches showed that chalcopyrite can be dissolved efficiently in the presence of thermophilic microorganisms such as Sulfolobus metallicus, Acidianus brierley or Metallosphera sedula[4-5]. There is considerable interest today in applying thermophiles to bioleach chalcopyrite in stirred tank reactors and in bioheap leaching. The Acidianus manzaensis (A. manzaensis) YN25 used in the experiments is a novel thermoacidophilic archaea in genus of Acidianus, firstly isolated from a fumarole in Manza by YOSHIDA et al in 2006[6], which could survive in the temperature range of 60-90 °C and pH range of 1.0-5.0. The cells were nearly regular cocci with an envelope comprised of the cytoplasmic membrane. It is a facultative autotroph capable of both anaerobic and aerobic growth, and it is able to oxidize ferrous iron and sulfur compounds to obtain energy for growth. Such energetic metabolism is responsible for metal solubilization. A pure culture of A. manzaensis YN25 was isolated from an acid hot spring in Yunnan Province, China, and preserved in our lab. The experiment done in our lab demonstrated that the strain YN25 possesses a prospective bioleaching potential.
Most leaching bacteria grow attached to surfaces of mineral sulfides and form biofilms that have well-developed community structures, with mechanisms for the delivery of nutrients and the disposal of waste products. There are indications that attachment to metal sulfides does not occur randomly. For example, atomic force microscopy (AFM) images demonstrate that cells of At. ferrooxidans preferentially (>80%) attach to sites with visible imperfection changes on surface[7]. This means that the bacteria maintain a close contact with the sulfide interface in order to dissolve it. In the past, attachment of bacteria to sulfide surfaces and the enhanced rates of dissolution of the sulfides were used as evidences that bioleaching proceeded via a direct mechanism (enzymatic attack). The search for evidence to support this hypothesis has resulted in innovative studies on the role of attachment in bioleaching[8]. It is proposed that the term “contact” leaching should be used in place of “direct” leaching because it describes the association of bacteria with a surface rather than the meaning of attack[9]. And some studies showed that sulphide minerals can be biologically leached by non- contact, contact or cooperative leaching mechanisms[10].
The aim of this study is to investigate the contribution of contact and non-contact cells of A. manzaensis YN25 to the bioleaching of chalcopyrite, and then whether the chalcopyrite is leached by A. manzaensis YN25 primarily in contact, non-contact or cooperative leaching mechanism is detected.
2 Experimental
2.1 Bacterial strain and growth conditions
The pure culture of A. manzaensis YN25 used in the experiments was isolated and conserved in our laboratory, and it was grown at 65 °C in medium 9K (without Fe2+) consisting of the following compounds: (NH4)2SO4 3.0 g/L, K2HPO4 0.5 g/L, MgSO4·7H2O 0.5 g/L, KCl 0.1 g/L, Ca (NO3)2 0.01 g/L, and yeast extract 0.2 g/L. And the initial pH was adjusted to 1.5. The inoculum was obtained by centrifugation and washed three times in acidified distilled water (pH 1.5). The cells then were suspended in basal salts medium without energy sources.
2.2 Mineral
The chalcopyrite used in the experiments was ground and passed through a sieve with a pore size of 75 ?m. The mineral sample mainly consisted of 90.4% chalcopyrite, 4.7% pyrite and 4.9% silicon dioxide.
2.3 Bioleaching experiments
Bioleaching experiments were carried out in a modified shake flask (Fig.1). The flask was composed of two parts: a big cylinder with a small cylinder in it. The end of the small cylinder was sealed with a 0.15 ?m millipore filter, which was immersed in the solution of the big cylinder.
1 g chalcopyrite particles were added in the big cylinder that contained 100 mL basal medium, and 40 mL basal medium was added in the small cylinder. Microorganisms were inoculated to get a cell population of 5×107 cells/mL. The microorganisms in the small cylinder could not reach the surface of chalcopyrite, but

Fig.1 Scheme of modified shake flask
were involved in the chemical process of the solution. The microorganisms in the big cylinder could contact the chalcopyrite, parts of which adsorb on the surface of chalcopyrite particles, and the others are planktonic cells. The planktonic cells have the chance to contact the surface of chalcopyrite particles. Thus, in this work, the microorganisms in the big cylinder are defined as the contact cells, while the microorganisms in the small cylinder are defined as the non-contact cells. Flasks were maintained at 65 °C and shaken at 150 r/min. Distilled water was added to the flask in order to compensate for evaporation losses. The experiments were carried out under three different conditions: experiment A, both of the cylinders were inoculated with A. manzaensis YN25; experiment B, only the small cylinder was inoculated with A. manzaensis YN25; experiment C, neither of the cylinders was inoculated.
2.4 Analysis methods
Redox potential and pH were measured daily, while the levels of copper and iron in the solution were analyzed by atomic absorption spectrophotometry every two days. The cell concentration in solution was determined by direct counting using hemocytometer under an optical microscope. Once the experiments were finished, the cells grown in the big cylinder and small cylinder were filtered through filter paper to remove cells from the suspended solid materials, respectively. The liquid containing the cells was then centrifuged and the cell pellet was obtained. So, the cells obtained from the big cylinder are the planktonic cells that have the chance to contact the surface of chalcopyrite particles. It was washed three times in dilute sulfuric acid (pH 1.5) in order to remove any trapped ions and freeze-dried so as to record the FT-IR spectra. The cell pellets were freeze-dried to be powders, and the quantity of powders which were added into the FT-IR instrument was the same every time. Thus, the cell population in the cylinder would not influence the intensities of the FT-IR peaks. And the leached residues were filtered and dried using a freeze drier. The morphological feature and chemical composition of the leached residues were analyzed by SEM and XRD, respectively.
3 Results and discussion
3.1 Bioleaching experiments
As shown in Fig.2, after 15 days of leaching, the maximum copper dissolution (about 620 mg/L) was reached in experiment A, in which the inoculated microorganisms were able to contact the surface of chalcopyrite. In experiment B where the inoculated microorganisms could not contact the surface of chalcopyrite, the copper dissolution was about 48% that in experiment A. The copper dissolution of experiment C was the least, only 166 mg/L. It was not inoculated in experiment C, and the chalcopyrite was slowly leached by H+ and O2 according to reaction (1)[2].
2CuFeS2+4H++O2→2Cu2++4S0+2Fe2++2H2O (1)
By comparing the results of experiments B and C, we can conclude that the non-contact A. manzaensis YN25 can also enhance the chalcopyrite dissolution. And by comparing the results of experiments A and B, we can conclude that the A. manzaensis YN25 has much more contribution to the dissolution of chalcopyrite if the microorganisms have the chance to contact the chalcopyrite. So, it is indicated that the leaching of the chalcopyrite is the cooperative action of the contact and non-contact A. manzaensis YN25, but the contact A. manzaensis YN25 is more important.

Fig.2 Copper dissolution during chalcopyrite leaching
As shown in Fig.3, the solution pH of experiments A and B increased to about 1.6 then started to decrease after 2 days, while the solution pH of experiment C increased all the time to about 2.4. Elemental sulfur was produced in the leaching of chalcopyrite, which may suspend as free aggregates and crystals, or can form a layer on the metal sulfide surface[11]. Then, some elemental sulfur was diffused into the small cylinder, which was oxidized to sulfuric acid. Compared with experiment A, less H+ production in experiment B should lead to a much higher pH value; however, such phenomenon would be weakened by the less H+ consumption because of the less effective leaching process (reaction (1)). Consequently, the decline of pH value in experiment B was also detected as well as that observed in experiment A. As a result, the two experiments presented close pH values. Chalcopyrite was leached in experiment C according to reaction (1). Since there was no H+ produced, the solution pH of experiment C increased all the time.

Fig.3 pH changes during bioleaching of chalcopyrite
As shown in Fig.4, the solution redox potential of experiment A reached about 570 mV (vs Ag/AgCl) at the end of the experiments, and the solution redox potential of experiment B only reached about 480 mV (vs Ag/AgCl), but the solution redox potential of experiment C decreased all the time. Solution redox potential mainly depends on the ratio of ρ(Fe3+)/ρ(Fe2+). Fe3+ was consumed for the oxidation of chalcopyrite, but Fe2+ could not be oxidized to Fe3+ as there was no A. manzaensis YN25 in experiment C, so the solution redox potential of experiment C decreased.

Fig.4 Solution redox potential changes during bioleaching of chalcopyrite
In experiment A, the cell population in the small cylinder increased during the first 4 days; but in experiment B, the cell population in the small cylinder decreased all the time. The reason is that there was no Fe2+ added in the leaching culture medium, and the microorganisms could not contact chalcopyrite in experiment B, so the energy resources (Fe2+ and S) were produced slowly according to reaction (1). Then, there was not enough energy, the growth rate of cells was slow, and maybe some cells adsorb on the wall of the cylinder and millipore filter. Therefore, the cell population in the solution of experiment B decreased all the time. But in experiment A, A. manzaensis YN25 in the small cylinder could grow out of the oxidation of some soluble species produced as intermediate compounds which mainly derived from chalcopyrite dissolution by microorganisms grown in the big cylinder:
3S0+2O2+2H2O![]() SO32-+S2O32-+4H+ (2)
SO32-+S2O32-+4H+ (2)
CuFeS2+4Fe3+→Cu2++2S0+5Fe2+ (3)
Fe2++H++1/4O2![]() Fe3++1/2H2O (4)
Fe3++1/2H2O (4)
3.2 FT-IR studies
FT-IR spectra of A. manzaensis YN25 cells grown in the small cylinder and big cylinder are shown in Fig.5. Both of the spectra show similar absorption features, although the intensities of the absorption bands are different.
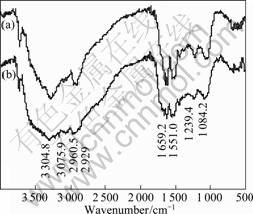
Fig.5 FT-IR spectra of A. manzaensis YN25: (a) Cells grown in small cylinder; (b) Cells grown in big cylinder
The bands were assigned according to previous reports[12-13]. The bands at 2 960 cm-1 and 2 929 cm-1 characterize asymmetric —CH3 stretching and asymmetric —CH2 stretching, respectively. The bands near 3 305 cm-1 and 3 076 cm-1 are assigned to asymmetric and symmetric stretching of the —NH2 group. The intense band at 1 659 cm-1 could be very sharp, indicating the presence of an amide group (amide I band). The bands at 1 551 cm-1 characterize —NH bending of the secondary amide group (—CONH). The band at 1 239 cm-1 is due to —CH3 wagging modes. The band near 1 084 cm-1 arises due to —CH3 rocking and —CH2 wagging modes. Thus, the FT-IR spectra obtained showed the presence of CH2, CH3, NH, NH2 and CONH groups on the surface of all the grown cells. The groups obtained are the groups present in any protein molecule. So, these data indicate that proteins existed on the surface of A. manzaensis. As revealed from the spectra, the intensities of all the absorbance peaks in the cells that could contact the chalcopyrite are larger than those that could not contact the chalcopyrite. So, the cells that could contact the chalcopyrite secrete more protein than those that could not contact the chalcopyrite on the cell surface. Since protein molecules are hydrophobic, it can be speculated that, the cells that can contact the chalcopyrite are more hydrophobic than the cells that cannot contact the chalcopyrite. These results suggest that under different growth conditions, the differences in the content of protein secreted by microorganisms may alter the surface characteristics of the cells. And the results are similar to the finding of SHARMA et al[12] and NATARAJAN and DAS[14].
3.3 Surface properties of leached residues
SEM images of the chalcopyrite before the experiment and the leached residues are shown in Fig.6. From Fig.6(a), we could see that the chalcopyrite was heavily etched in experiment A, and there were lots of cells attached in the etched pits (the arrows show the microorganisms). From Figs.6(b) and (c) we found that the surfaces of the leached residues were smooth and there were some cracks on it. It can be speculated that there were passivating layers on the surfaces of the leached residues in experiments B and C which were supposed to be sulfur or jarosite. This was verified by the X-ray diffraction results of the leached residues (Fig.7). The jarosite was not detected in the leached residues of all these experiments. This may be due to the very low pH values in the culture systems, which resulted in little jarosite precipitation[15-16]. But, the sulfur was detected in the leached residues of experiments B and C except experiment A, so the layer on the leached residues of the experiments B and C are composed of sulfur. It was not inoculated in experiment C, where the chalcopyrite was slowly leached by H+ and O2 according to reaction (1). Consequently, the elemental sulfur was accumulated and formed a layer on the surface of chalcopyrite. From this, we can conclude that the layer composed of sulfur could be removed by contact cells, but non-contact cells in the small cylinder could not do this. Consequently, elemental sulfur may accumulate in
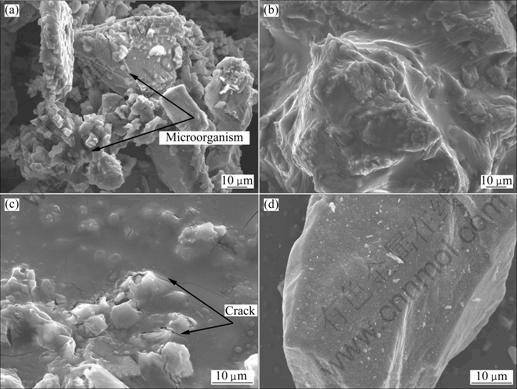
Fig.6 SEM images of leached residues: (a) Experiment A; (b) Experiment B; (c) Experiment C; (d) Chalcopyrite before experiment
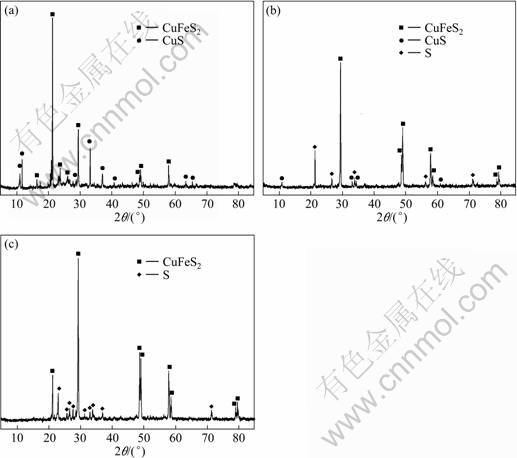 Fig.7 XRD patterns of leached residues: (a) Experiment A; (b) Experiment B; (c) Experiment C
Fig.7 XRD patterns of leached residues: (a) Experiment A; (b) Experiment B; (c) Experiment C
4 Conclusions
1) It was found that the leaching of chalcopyrite was the cooperative action of the contact and non-contact A. manzaensis YN25. And the experimental results showed that the non-contact A. manzaensis YN25 could also enhance the chalcopyrite dissolution, but the A. manzaensis YN25 that could contact the surface of minerals played a more important role in the bioleaching process of chalcopyrite.
2) The analysis of leached residues indicated that the passivation of chalcopyrite concentrate was mainly due to the elemental sulphur layer on the mineral surface, other than the jarosite. The surface passivating layer of sulfur could be removed only when the A. manzaensis YN25 could contact the surface of the chalcopyrite.
3) The amount of protein on the surface of A.manzaensis YN25 was higher when the cells could contact chalcopyrite, which endowed the cells with more hydrophobicity.
References
[1] HALLBERG K B, JOHNSON D B. Biodiversity of acidophilic prokaryotes [J]. Advances in Applied Microbiology, 2001, 49: 37-84.
[2] PETERSEN J, DIXON D G. Thermophilic heap leaching of chalcopyrite concentrate [J]. Minerals Engineering, 2002, 15(11): 758-777.
[3] RODRIGUEZ Y, BALLESTER A, BLAZQUEZ M L, GONZALEZ F, MUNOZ J A. New information on the chalcopyrite bioleaching mechanism at low and high temperatures [J]. Hydrometallurgy, 2003, 71(1/2): 47-56.
[4] MIKKELSEN D, KAPPLER U, WEBB R I, RASCH R, MCEWAN A G, SLY L I. Visualisation of pyrite leaching by selected thermophilic archaea: Nature of microorganism-ore interactions during bioleaching [J]. Hydrometallurgy, 2007, 88: 143-153.
[5] HARNEIT K, G?KSEL A, KOCK D, KLOCK J H, GEHRKE T, SAND W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans [J]. Hydrometallurgy, 2006, 83: 245-254.
[6] YOSHIDA N, NAKASATO M, OHMURA N, ANDO A, SAIKI H, ISHII M, IGARASHI Y. Acidianus manzaensis sp. nov., a novel thermoacidophilic archaeon growing autotrophically by the oxidation of H2 with the reduction of Fe3+ [J]. Current Microbiology, 2006, 53(5): 406-411.
[7] EDWARDS K J, RUTENBERG A D. Microbial response to surface microtopography: The role of metabolism in localized mineral dissolution [J]. Chemical Geology, 2001, 180(1/4): 19-32.
[8] ROHWERDER T, GEHRKE T, KINZLER K, SAND W. Bioleaching review (part A): Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation [J]. Applied Microbiology Biotechnology, 2003, 63(3): 239-248.
[9] TRIBUTSCH H. Direct versus indirect bioleaching [J]. Hydrometallurgy, 2001, 59(2/3): 177-185.
[10] KINNUNEN P H M, HEIMALA S, RIEKKOLA V M L, PUHAKKA J A. Chalcopyrite concentrate leaching with biologically produced ferric sulphate [J]. Bioresource Technology, 2006, 97(14): 1727-1734.
[11] MCGUIRE M M, EDWARDS K J, BANFIELD J F, HAMERS R J. Kinetics, surface chemistry, and structural evolution of microbially mediated sulfide mineral dissolution [J]. Geochimica et Cosmochimica Acta, 2001, 65(8): 1243-1258.
[12] SHARMA P K, DAS A, HANUMANTHA RAO K, FORSSBERG K S E. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions [J]. Hydrometallurgy, 2003, 71(1/2): 285-292.
[13] SHARMA P K, HANUMANTHA R K. Surface characterization of bacterial cells relevant to the mineral industry [J]. Minerals and Metallurgical Processing, 2005, 22(1): 31-37.
[14] NATARAJAN K A, DAS A. Surface chemical studies on ‘Acidithiobacillus’ group of bacteria with reference to mineral flocculation [J]. International Journal of Mineral Processing, 2003, 72: 189-198.
[15] POGLIANI C, DONATI E. The role of exopolymers in the bioleaching of a non-ferrous metal sulphide [J]. Journal of Industrial Microbiology and Biotechnology, 1999, 22(2): 88-92.
[16] KLAUBER C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution [J]. Mineral Processing, 2008, 86(1/2/3/4): 1-17.
[17] FOWLER T A, CRUNDWELL F K. Leaching of zinc sulfide by Thiobacillus ferrooxidans: Bacterial oxidation of the sulfur product layer increases the rate of zinc sulfide dissolution at high concentrations of ferrous ions [J]. Applied and Environmental Microbiology, 1999, 65(12): 5285-5292.
Foundation item: Project(50621063) supported by the National Natural Science Foundation of China; Project(DYXM-115-02-2-07) supported by the China Ocean Mineral Resources Research and Development Association; Project(200805032) supported by the State Oceanic Administration of China
Corresponding author: ZHOU Hong-bo; Tel: +86-731-88877216; Email: zhouhb@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(09)60405-2


