
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1866-1871
Formation of precipitates and recrystallization resistance in Al-Sc-Zr alloys
JIA Zhi-hong1,2, Jostein R?YSET3, Jan Ketil SOLBERG2, LIU Qing1
1. School of Materials Science and Engineering, Chongqing University, Chongqing 400044, China;
2. Department of Materials Science and Engineering,
Norwegian University of Science and Technology, NO-7491, Trondheim, Norway;
3. Hydro Aluminium Research and Technology Development, NO-6600, Sunndals?ra, Norway
Received 10 November 2011; accepted 20 June 2012
Abstract:
Al-x%Sc-0.11%Zr alloys (x=0, 0.02, 0.05, 0.08, 0.11, 0.15) were produced by a chill cast procedure. The effect of Sc content on the precipitation of Al3(Sc,Zr) during heat treatment at 475 ℃ for 12 h was studied. Nucleation, precipitation and distribution of Al3(Sc,Zr) precipitates were found to be strongly related to the Sc content. With increasing the Sc content, the average radius of the precipitates decreases, while the number density of the precipitates increases, as investigated by transmission electron microscopy (TEM). The distribution of the precipitates becomes more and more homogeneous when the Sc content is increased. The recrystallization resistance of samples that was 90% cold rolled and isothermal annealed for half an hour in the temperature range of 200-600 ℃ was investigated. The results show that the recrystallization temperature varies from 250 ℃ for the alloy without Sc to about 600 ℃ for the alloy containing 0.15% Sc because of the high density of Al3(Sc,Zr) precipitates.
Key words:
aluminium alloy; precipitate; recrystallization resistance;
1 Introduction
A tremendous amount of research has been carried out on Sc-containing Al alloys over the last 20 years, owing to the improved properties that can be obtained by a Sc addition [1-8]. One of the most important advantages is related to the formation of a dense and homogeneous distribution of precipitates during thermomechanical processing at elevated temperatures, which in turn leads to an efficient control of the microstructure of the alloy [9,10]. The precipitates are of the Al3Sc phase, which is the equilibrium phase of the Al-Sc phase diagram [1]. It is well known that adding Sc in combination with Zr leads to a higher temperature stability of the precipitates (slower rate of coarsening) as compared with a Sc addition alone. The Al3Sc phase can dissolve a considerable amount of Zr when Sc and Zr are added in combination. The precipitates are frequently denoted as Al3(Sc1-xZrx). It has been discovered that, due to the faster diffusion rate of Sc as compared with Zr, the formation of these precipitates starts with almost pure Al3Sc whereas the Zr content gradually increases during growth of the precipitates, leading to a core/shell structure of the precipitates [11-13].
The high price of Sc is prohibitive for extended commercial applications of Sc-containing aluminium alloys. At present, the use is limited to high-end sporting equipment and to a few aerospace applications. In order to draw the benefit of Sc addition in other product ranges, it is necessary to minimize the associated cost by establishing the minimum Sc content necessary for obtaining the desired effect. The aim of the present investigation is to determine the minimum Sc content needed for achieving a dense and homogeneous distribution of Al3(Sc1-xZrx) precipitates in an Al-Sc-Zr alloy.
2 Experimental
2.1 Material preparation
Six Al-Sc-Zr alloys with different Sc contents were prepared. Fe and Si are inevitable impurities in commercial Al alloys, and one therefore chose aluminium-based metal with a small content of Fe and Si for producing these alloys. Sc and Zr were added as Al-10%Sc and Al-10%Zr master alloys, respectively. The aluminium ingot was first melted and well stirred at 820 ℃ and the Sc and Zr master alloys covered with aluminium foil were subsequently added into the melt and kept on the melt surface till being melted, which is to reduce the oxidation of Sc and especially Zr. Three billets of d40 mm×150 mm for each alloy were cast in a water-cooled mould. The Zr, Fe and Si contents were determined with optical emission spectrograph calibrated against certified standards, whereas due to lack of standards the Sc content was measured with standardless X-ray fluorescence spectroscopy. Table 1 lists the results of the measurements.
Table 1 Chemical compositions of series alloys
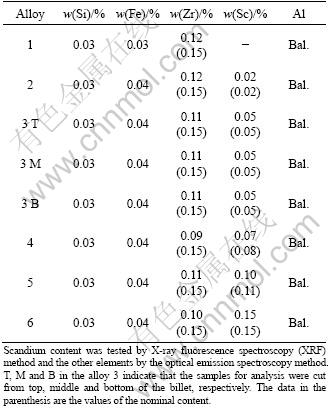
The measured Sc content is in good consistence with the nominal content. The measured Zr content of 0.11% is invariable through the series alloys, which is slightly lower than the nominal content, 0.15% due to its unavoidable oxidation during adding to the melting. The impurities of Si and Fe elements in all the alloys are invariable. The homogeneous distribution of each alloying element throughout the billets is revealed by investigating the different parts of alloy 3.
2.2 Hardness measurements
Vickers microhardness measurements were performed on metallographically polished sections of the alloys using a load of 9.8 N and time of indentation of 15 s. Each reported hardness value is an average of seven measurements.
2.3 Heat treatment procedures
A set of as-cast specimens were heated from room temperature in an air-circulation furnace at a rate of 50 ℃/h and kept at 475 ℃ for 12 h for investigation of precipitates. All specimens were water-quenched when removed from the furnace.
The samples for recrystallization resistance study were 90% cold rolled and isothermal annealed for half an hour in the temperature range of 200-600 ℃ with an interval of 50 ℃ after precipitation annealing. The isothermal annealing was carried out in a sand bath at 200 ℃ and 250 ℃, and in a salt bath at 300-600 ℃.
2.4 Microstructural characterization
A JEOL2010 transmission electron microscope (TEM) with an accelerating voltage of 200 kV was used for investigating the general microstructure and the distribution of Al3Zr or Al3(Sc,Zr) precipitates. Thin foils for TEM were prepared by cutting sample pieces from the heat treated materials and by mechanically grinding them down to ~100 μm. Discs with 3 mm in diameter were punched and dimpled down to 60 μm, followed by twin-jet electropolishing at 20 V (Struers Tenupol-5). A solution of 1/3 (volume fraction) nitric acid and 2/3 methanol at -30 ℃ was applied during the electropolishing.
3 Results
3. 1 Effect of scandium content on precipitation of Al3(Sc, Zr) precipitates
As understood from the scandium-free alloys, zirconium diffuses very slowly during heat treatment, leading to a long time for precipitation of the Al3Zr precipitate. In order to study the effect of scandium on the Al3(Sc,Zr) precipitates by TEM, all as-cast specimens from six alloys were annealed at 475 ℃ for 12 h although it is maybe not necessary for the higher scandium-containing alloys. No precipitate was observed from alloy 1, the binary Al-Zr alloy at the present condition. Figure 1 shows TEM images of alloy 2 containing only 0.02% Sc. Both diffractions from Al matrix and the precipitate are shown in Fig. 1(a). The location of the diffraction spots from the precipitates at the centre of two diffraction spots from Al matrix indicates the coherency between the Al matrix and the Al3(Sc,Zr) precipitates. By selecting the only one small diffraction dot indicated in Fig. 1(a) the dark field image was taken and the Al3(Sc,Zr) precipitates were observed clearly (Fig. 1(b)). The precipitates have an average diameter of 45 nm. The bright field image from the same area shown in Fig. 1(b) shows precipitates located in dislocation region (Fig. 1(c)).
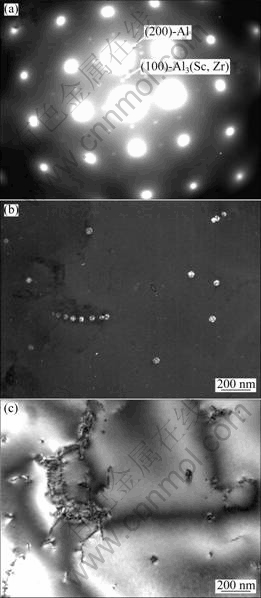
Fig. 1 TEM images of Al3(Sc,Zr) precipitates in alloy 2 annealed at 475 ℃ for 12 h: (a) Diffraction pattern along [100] zone axis; (b) TEM dark field image showing Al3(Sc,Zr) precipitates; (c) TEM bright field image from same area as shown in (b) showing precipitation related to dislocations
Figure 2 shows TEM dark field images of Al3(Sc,Zr) precipitates from the samples of alloys 3-6 after annealing at 475 ℃ for 12 h. Al3(Sc,Zr) precipitates with a higher number density were found in alloy 3 compared with alloy 2, and the average diameter of the precipitates is 17 nm, much smaller than those in alloy 2. With further increasing scandium content, the number density of Al3(Sc,Zr) precipitates increases evidently, while the average radius of the precipitates is at the same level if considering the measuring error, as indicated in Fig. 3. Another feature is that a homogeneous distribution of Al3(Sc,Zr) precipitates can be obtained when Sc concentration is equal to or more than 0.05%, in spite of the dislocation-related precipitation of Al3(Sc,Zr) precipitates still existing in the alloys, as indicated in Fig. 2(d). No Al3(Sc,Zr) precipitates are observed at the right top and the left bottom in Fig. 2(a) due to serious bend contrast, leading to unobservable Al3(Sc,Zr) precipitates at those areas.
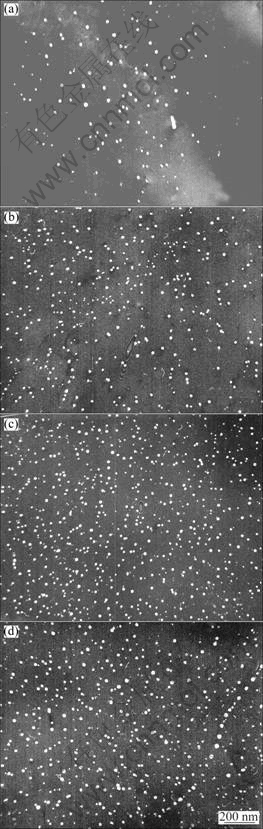
Fig. 2 Precipitation of Al3(Sc,Zr) precipitates annealed at 475 ℃ for 12 h: (a) Alloy 3; (b) Alloy 4; (c) Alloy 5; (d) Alloy 6
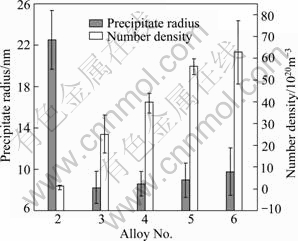
Fig. 3 Relationship between precipitate radius and number density with Sc content in alloys
3.2 Effect of Al3(Zr,Sc) precipitates on recrystalliza- tion resistance of alloys after 90% cold rolling
The softening process of the alloys as a function of temperature is shown in Fig. 4. The alloys were first heat treated at 475 ℃ for 12 h to form the Al3(Zr,Sc) precipitates, and subsequently cold rolled 90%, following by isothermal annealing at different temperatures for half an hour. Generally, the recrystallization temperature rises from alloy 1 to alloy 6, i.e. with increase of the Sc content. The binary Al-Zr alloy (alloy 1) was recrystallized completely at 350 ℃. However, there is even not a complete recrystallization for the Al-Zr-Sc alloy with only 0.02% Sc (alloy 2) up to 500 ℃. The alloys 3 and 4 have a similar starting temperature of recrystallization, i.e. 500 ℃, judging from the hardness curves. The optical microscopy investigations from the anodized specimen of the alloy 5 reveal that there is no recrystallization at 250 ℃ (Fig. 5(a)), starting recrystallization at 550 ℃ (Fig. 5(b)), and a complete recrystallization at 600 ℃ (Fig. 5(c)). The smooth decrease of the hardness in the alloys before recrystallization is attributed to the recovery of the alloys during isothermal annealing. The maximum recrystallization resistance comes from the alloy 6 which is not completely recrystallized even at 600 ℃.
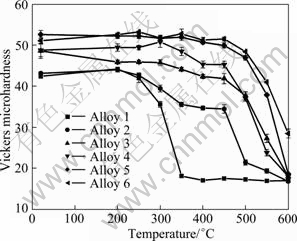
Fig. 4 Softening curves of six alloys precipitation heat treated at 475 ℃ for 12 h, followed by 90% cold rolling and isothermal annealing for 0.5 h
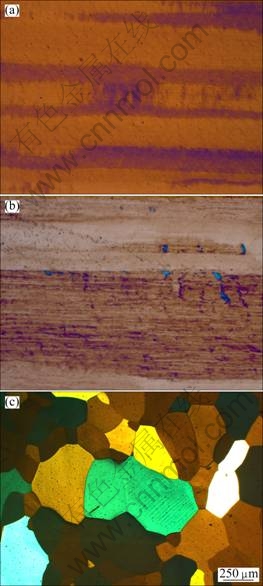
Fig. 5 Optical micrographs of cold-rolled alloy 5 after isothermal annealing for 0.5 h: (a) 250 ℃, no recrystallization; (b) 550 ℃, starting recrystallization; (c) 600 ℃, complete recrystallization
4 Discussion
TEM investigations of the precipitates, as compared with alloy 1, alloys 2-6, manifest that addition of Sc could promote the decomposition of supersaturated Zr in solid solution. It is known that Zr and Sc elements segregate in the dendrite centre and edge, respectively, in solidifying process [14]. The diffusion rate of Sc solute atoms is much faster than Zr. During the precipitation heat treatment, Sc atoms may first diffuse to sites with structural defects, like dislocation. Because of low energy barrier, the nucleation of Al3Sc can be started at such region, which further attracts Zr atoms to form the core/shell Al3(Zr,Sc) precipitate. For the alloy with low content of Sc, for instance, alloy 2 with 0.02% Sc, the limited nucleation is mainly restricted to those defect sites having low energy barrier, which could grow up easily with enough solute atoms surrounding during heat treatment period, and as a result, there will be large precipitates observed as shown in Fig. 1. When more Sc is added in the alloy, nucleation will occur anywhere including defect sites, namely, homogenous nucleation is dominating. In the case, all nuclei will compete with each other for the limited solute atoms of Sc and Zr, leading to homogenous distribution and small average size of the Al3(Zr,Sc) precipitate, as seen in Fig. 2. In addition, the formation of the core/shell precipitate slows down the coarsening rate of the precipitate at elevated temperatures, as compared with the Al3Sc precipitates.
The recovery and recrystallization process is mainly related to the movement and coalescence of dislocations and grain boundaries. The hardening mechanism depends on the interaction of moving dislocations with the precipitates. There are two ways of the interaction between dislocations and the precipitates, i.e., precipitate cutting mechanism and precipitate bypass mechanism, which is mainly related to the precipitate size. Roughly speaking, when the precipitate is less than 5 nm in diameter, the precipitate cutting mechanism will be dominated; otherwise, it will be mainly the bypass mechanism. TEM images from the alloy 2 in Fig. 1 do not show homogeneous distribution and high density of the Al3(Zr,Sc) precipitates. However, a large improvement of recrystallization resistance is detected (Fig. 4). There are two possible reasons. One is that there is homogeneous and dense Al3(Zr,Sc) precipitates with very small size, which are not observed under the present TEM conditions, hardening the material by the precipitate cutting mechanism. Another possibility is Sc solute atoms dissolving in solid solution and causing hardening. It needs more experimental work to clarify the real hardening mechanism. For the alloy containing over 0.02% Sc, the precipitation hardening is the bypass mechanism. It is pointed out that Al3Sc precipitates prevent recrystallization up to temperatures of 400-450 ℃, and at higher temperatures, the precipitates coarsen considerably and the microstructure is partially recrystallized [15]. From the detailed investigations in this work, it can be seen that the cold rolled alloy 6 can keep uncrystallization at a temperature up to 550 ℃. This confirms that the Al3(Zr,Sc) precipitate with the Zr atoms as shell has a low coarsening rate making it stable at elevated temperatures.
5 Conclusions
1) Sc addition may promote the decomposition of supersaturated Zr from the solid solution, and form the core/shell Al3(Zr,Sc) precipitates. Homogeneous and dense distribution of the Al3(Zr,Sc) precipitates can be reached by adding 0.05% Sc or more. The number density of the precipitates is proportional to the Sc content.
2) Addition of 0.02% Sc effectively raises an initial temperature of recrystallization as compared with the Al-Zr binary alloy. Such good improvement of recrystallization resistance would be related to coherent Al3(Zr,Sc) precipitates efficiently pinning the movement of dislocations and grain boundaries.
References
[1] R?YSET J, RYUM N. Scandium in aluminium alloys [J]. Inter Mater Rev, 2005, 50(1): 19-44.
[2] DAI Xiao-yuan, XIA Chang-qing, PENG Xiao-min. Precipitation behavior of Al3(Sc,Zr) secondary particles in 7××× aluminum alloys during annealing [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(3): 451-455. (in Chinese)
[3] DAI Xiao-yuan, XIA Chang-qing, HUA Man-yu, PENG Xiao-min. Recrystallization of 7××× aluminum alloy containing scandium [J]. Trans Mat Heat Treat, 2010, 31(1): 132-136.
[4] HE Zhen-bo, PENG Yong-yi, YIN Zhi-min, LEI Xue-feng. Comparison of FSW and TIG welded joints in Al-Mg-Mn-Sc-Zr plates [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(8): 1685-1691.
[5] BOOTH-MORRISON C, DUNAND D C, SEIDMAN D N. Coarsening resistance at 400 ℃ of precipitation-strengthened Al-Zr-Sc-Er alloys [J]. Acta Mater, 2011, 59: 7029-7042.
[6] KARNESKY R A, DUNAND D C, SEIDMAN D N. Evolution of nanoscale precipitates in Al microalloyed with Sc and Er [J]. Acta Mater, 2009, 57: 4022-4031.
[7] LEFEBVRE W, DANOIX F, HALLEM H, FORBORD B, BOSTEL A, MARTHINSEN K. Precipitation kinetic of Al3(Sc,Zr) dispersoids in aluminum [J]. J Alloys Comp, 2009, 470: 107-110.
[8] FULLER C B, SEIDMAN D N. Temporal evolution of the nanostructure of Al(Sc,Zr) alloys: Part II-Coarsening of Al3(Sc1-xZrx) precipitates [J]. Acta Mater, 2005, 53: 5415-5428.
[9] SEIDMAN D N, MARQUIS E A, DUNAND D C D. Precipitation strengthening at ambient and elevated temperatures of heat-treatable Al(Sc) alloys [J]. Acta Mater, 2002, 50: 4021-4035.
[10] RIDDLE Y W, SANDERS T H Jr. A study of coarsening, recrystallization, and morphology of microstructure in Al-Sc-(Zr)- (Mg) alloys [J]. Metall Mater Trans A, 2004, 35: 341-350.
[11] CLOUET E, LAE L, EPICIER T, LEFEBVRE W, NASTAR M, DESCHAMPS A. Complex precipitation pathways in multicomponent alloys [J]. Nature Materials, 2006, 5: 482-488.
[12] FORBORD B, LEFEBVRE W, DANOIX F, HALLEM H, MARTHINSEN K. Three dimensional atom probe investigation on the formation of Al3(Sc,Zr)-dispersoids in aluminium alloys [J]. Scripta Mater, 2004, 51: 333-337.
[13] TOLLEY A, RADMILOVIC V, DAHMEN U. Segregation in Al3(Sc,Zr) precipitates in Al–Sc–Zr alloys [J]. Scripta Mater, 2005, 52: 621-625.
[14] KNIPLING K E, KARNESKY R A, LEE C P, DUNAND D C, SEIDMAN D N. Precipitation evolution in Al–0.1Sc, Al–0.1Zr and Al–0.1Sc–0.1Zr (at.%) alloys during isochronal aging [J]. Acta Mater, 2010, 58: 5184-5195.
[15] DRITS M E, PAVLENKO S G, TOROPOVA L S, BYKOV Y G, BER L B. Recrystallization of Al-Sc alloys [J]. Sov Phys Dokl, 1981, 26(3): 344-346.
Al-Sc-Zr合金中析出相的形成及再结晶抑阻
贾志宏1,2, Jostein R?YSET3, Jan Ketil SOLBERG2, 刘 庆1
1. 重庆大学 材料科学与工程学院,重庆 400044;
2. Department of Materials Science and Engineering,
Norwegian University of Science and Technology, NO-7491, Trondheim, Norway;
3. Hydro Aluminium Research and Technology Development, NO-6600, Sunndals?ra, Norway
摘 要:通过冷硬铸造法制备Al-x%Sc-0.11%Zr合金(x = 0,0.02,0.05,0.08,0.11,0.15),研究合金在475 ℃保温12 h热处理条件下不同钪含量对Al3(Sc, Zr)析出的影响。研究发现,Al3(Sc, Zr)析出相的形核、析出和分布与钪含量密切相关。透射电子显微镜研究表明,随着钪含量的增加,析出相的平均半径减小,密度增大,分布变得越来越均匀。将90%冷轧样品在200~600 ℃的温度区间等温退火0.5 h,再进行抗再结晶能力的研究。结果表明,未添加钪的合金的再结晶温度为250 ℃,而添加0.15%钪后形成的Al3(Sc, Zr)析出相使得再结晶温度升高到约600 ℃。
关键词:铝合金;析出相;再结晶抑阻
(Edited by LI Xiang-qun)
Foundation item: Project (CDJZR12130048) supported by the Fundamental Research Funds for the Central Universities of China; Project supported by the Research Council of Norway
Corresponding author: JIA Zhi-hong; Tel: +86-23-65102029; E-mail: zhihongjia@cqu.edu.cn
DOI: 10.1016/S1003-6326(11)61399-X
Abstract: Al-x%Sc-0.11%Zr alloys (x=0, 0.02, 0.05, 0.08, 0.11, 0.15) were produced by a chill cast procedure. The effect of Sc content on the precipitation of Al3(Sc,Zr) during heat treatment at 475 ℃ for 12 h was studied. Nucleation, precipitation and distribution of Al3(Sc,Zr) precipitates were found to be strongly related to the Sc content. With increasing the Sc content, the average radius of the precipitates decreases, while the number density of the precipitates increases, as investigated by transmission electron microscopy (TEM). The distribution of the precipitates becomes more and more homogeneous when the Sc content is increased. The recrystallization resistance of samples that was 90% cold rolled and isothermal annealed for half an hour in the temperature range of 200-600 ℃ was investigated. The results show that the recrystallization temperature varies from 250 ℃ for the alloy without Sc to about 600 ℃ for the alloy containing 0.15% Sc because of the high density of Al3(Sc,Zr) precipitates.


