
Synthesis of tetragonal BaTiO3 film on Ti substrate by micro-arc oxidation
PENG Ji-hua(彭继华), LI Wen-fang(李文芳), HAN Bing(韩 冰), HUANG Fang-liang(黄芳亮), DU Jun(杜 军)
School of Materials Science and Engineering, South China University of Technology,
Guangzhou 510640, China
Received 1 October 2007; accepted 30 June 2008
Abstract:
BaTiO3 films on Ti substrate were fabricated by alternative current(AC) and direct current(DC) micro arc oxidation (MAO). Microstructures of films were investigated by means of SEM, XRD and TEM. The results show that the amorphous phase and primitive cubic phase are the main phases in the films prepared by AC MAO. Even after being annealed at 1 200 ℃ for 8 h, only a few tetragonal phases can be observed in films prepared by AC MAO. However, tetragonal BaTiO3 phase can be produced by DC MAO directly. In the films prepared by DC MAO, a mixture of cubic phase and tetragonal phase is formed. After sparking spacious distribution, sparking duration and temperature gradient near sparking sites were taken into account, and a mechanism of synthesis of tetragonal BaTiO3 phase by DC MAO was proposed.
Key words:
micro-arc oxidation; peroskite; thin film; barium titanate; microstructure;
1 Introduction
Owing to its superior dielectric, ferroelectric, piezoelectric, pyroelectric and electro-optical properties, barium titanate is an important functional material in the electronics industry, for example, tunable microwave dielectrics[1-2] and integrated capacitors[3]. It is well- known that there is a Curie temperature. BaTiO3 is in the paraelectric state at the Curie temperature, and the ferroelectric state is below this temperature (about 120 ℃). However, the paraelectric cubic phase of BaTiO3 is usually the dominant phase at room temperature in the powder prepared by chemical solution process[4-7]. The post heat treatment is necessary to obtain tetragonal phase in BaTiO3 powder.
In order to push the ferroelectric BaTiO3 into practical application, many synthesis means were developed in the past decade. It is possible to obtain products with relative density of about 99% by means of self-propagation high temperature synthesis combined with spark-plasma sintering[8]. Nano-particle tetragonal barium titanate was directly produced by low temperature combustion synthesis process (LCS)[9]. The technique combining the conventional sol-gel process and hydrothermal methods (SGHT) was used to fabricate a pure BaTiO3 thin film[10]. Recently, it is reported that a less than 100 nm thin BaTiO3 film was built from nano-particle with 20 nm in diameter, and the dielectric constant of 85-90 was measured in this film[11].
It is known that micro-arc oxidation(MAO) is another feasible means to fabricate BaTiO3 films using barium hydroxide or barium acetate as the electrolyte, and this ceramic coating could improve corrosion resistance of titanium plate[12-14]. Cubic BaTiO3 was the main phase in the films prepared by MAO[12-16]. The stability of cubic BaTiO3 at room temperature was discussed in the aspects of critical size, surface depolarization[17] and constraint stress[18]. The objective of this work is to synthesize the tetragonal BaTiO3 film by MAO. The phase constituents in the films prepared by AC and DC MAO were studied, and the reason why tetragonal BaTiO3 films can be prepared by DC MAO was discussed.
2 ExperimentalThe titanium TA1 was used as the substrate materials in the present study, and it has a chemical composition (mass fraction, %) of N≤0.03, C≤0.05, H≤0.012, Fe≤0.20, O≤0.15 and Ti balance. Prior to coating deposition, the samples with a dimension of 40 mm×40 mm×2 mm were ground with abrasive paper up to 800 grits, degreased with alkaline solution, immersed in 10% HF solution for 120 s, and rinsed with distilled water, then dried in air at room temperature. The self-made MAO device consists of a high power supply unit, a stainless steel container that also serves as the counter electrode, a stirring and cooling system. A symmetry rectangular alternative current wave was used with frequency of 250 Hz and current density of 2-5 A/cm2. Electrolyte was made of 0.2 mol/L barium hydroxide, additives and distilled water. The micro-arc oxidization process was run for 5–20 min. The electrolyte temperature was controlled at 70-90 ℃. After anodization, some samples were closed in vessel, into which a few BaO was put. In the vessel, BaO was kept not to contact with the Ti substrate and the thin film, in order to avoid contamination of the sample during annealing. Annealing at various temperatures was run for 8 h followed by cooling in furnace.
A current density of 150 mA/cm2 was used in direct current micro-arc oxidation. Electrolyte consists of 0.5 mol/L barium hydroxide. During DC MAO, average electrolyte temperature of 57 ℃ was controlled. MAO treatment duration was in the range from 5 min to 20 min.
The phase constituents of the ceramic coatings were investigated by means of XRD (Philip X-pert). Micro- structure of coatings was studied by SEM (LEO-1530) and TEM (JEM-2000FX). The AC MAO TEM sample was made at University of Science and Technology Beijing, China, as follows: firstly, the coating film was scratched and fractured; secondly, the coating fragments fractured were wrapped in copper; at last, TEM samples were finished by mechanical grinding and ion sputtering. The DC MAO TEM sample was prepared and observed at Hong Kong City University, China.
3 Results3.1 Microstructure of film prepared by AC MAO
Fig.1(a) presents the SEM surface image of films prepared under AC micro-arc oxidation of current density 5 A/cm2, barium hydroxide 0.2 mol/L and hold time 12 min. On the surface, many craters with various sizes are distributed uniformly, and the larger size could be in micrometer magnitude. XRD patterns of films as- prepared and as-annealed are shown in Figs.1(b) and 1(c), respectively. With the AC micro-arc oxidation treat time increasing, the contents of amorphous phases decrease (Fig.1(b)). In the films as-prepared, constituent phases include amorphous and cubic phases. Annealed for 8 h in temperature range from 900 ℃ to 1 200 ℃, cubic phase of BaTiO3 is dominant (Fig.1(c)) in films. From the XRD pattern of the film annealed at 1 200 ℃ for 8 h, (002) around 2q=45? splitting is observed in Fig.1(c), but it is somewhat ambiguous. Because of the change of the ratio of c to a, splitting of (002) around 2q=45? is the characteristic of transformation from cubic phase to tetragonal phase. This indicates that a few tetragonal phases of BaTiO3 probably exist in the films as-annealed.

Fig.1 Surface morphology (a), XRD patterns of films prepared at 5 A/cm2 (b) and films annealed for 8 h at different tempera- tures (c)
Fig.2(a) shows the bright TEM image of the film prepared in 0.2 mol/L Ba(OH)2 solution for 3 min under current density of 5 A/cm2. The zone A in Fig.2(a) is anamorphous phase, from which the selected area diffraction(SAD) is shown in the up-left corner. In Fig.2(a), zones B and D can be indexed as primitive cubic BaTiO3 phase (PDF 75-0213), and zone C can be determined to be hexagonal phase (PDF 82-1175). The microstructure of a fine equiaxial grain is shown in Fig.2(b). The up-left corner inlet presents the SAD from grain “1” in Fig.2(b). From the SAD patterns, grain “1” and zone “2” are determined to be cubic BaTiO3, meanwhile, zones “4” and “5” are referred to hexagonal BaTiO3. Comparing Fig.2(a) with Fig.2(b), it shows crystallization of amorphous phase and grain growth during annealing.

Fig.2 TEM bright field(BF) images of films: (a) As-prepared and SAD patterns of zone A; (b) As-annealed and SAD pattern of grain “1”
3.2 Microstructure of film prepared by DC MAO
Compared with Fig.1(a), the film surface prepared by DC micro-arc oxidation (Fig.3(a)) is much rougher than that prepared by AC MAO. From the XRD patterns (Fig.3(b)), two main constituent phases are included in the films prepared by DC MAO: BaCO3 and tetragonal BaTiO3. In the XRD patterns, the (002) around 2q=45? is observed to split into two peaks, indicating the tetragonal BaTiO3 phase. Appearance of BaCO3 is probably responsible for the reaction between CO2 and Ba(OH)2[14], in which CO2 comes from air, and Ba(OH)2 comes from the residual electrolyte solution on film surface. Most probably, the presence of BaCO3 is contributed to no rinsing and drying immediately after MAO.
Ba(OH)2+CO2→BaCO3+H2O (1)
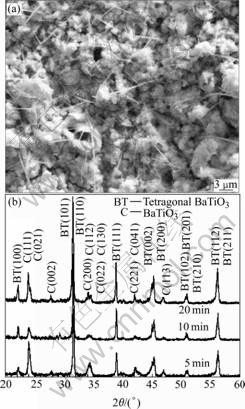
Fig.3 SEM surface image (a) and XRD patterns (b) of films prepared at 150 mA/cm2
Selected area diffraction(SAD) pattern and HREM images of film prepared by DC MAO are presented in Fig.4. Diffraction pattern in Fig.4(a) can be determined to be cubic BaTiO3 phase, in which a=0.405 7 nm (PDF 75-0215). Analysis from Fig.4(b) proves that tetragonal phase of BaTiO3 exists in the film prepared by direct current micro-arc oxidation. According to PDF 74-1965, A, B and C in SAD (Fig.4(b)) can be indexed as (100), (010) and (101) respectively. In tetragonal BaTiO3 phase, cell parameters are: a=0.399 2 nm and c=0.403 4 nm. So it can be concluded that the film prepared by DC MAO is a mixture of paraelectric (cubic) and ferroelectric (tetragonal) phases. Spontaneous polarization and hysteresis, indicating ferroelectric behavior in the film prepared by DC MAO, are shown in electric field dependant polarization measurements and will be published soon.

Fig.4 TEM images of films prepared by DC MAO: (a) Cubic phase; (b) Tetragonal phase
4 DiscussionIt is well-known that the passive film of TiO2 formed on Ti substrate is critical for sparking. When the dense passive film of TiO2 formed on Ti substrate in alkaline solution, higher voltage is needed to break down this dielectric layer. In the center of sparking zone, temperature is estimated to be high enough to melt the oxide[19]. Consequently, this creates a condition to meet the requirement of the following reaction:
Ba(OH)2+TiO2→BaTiO3+H2O (2)
In the center of sparking zone, the primary BaTiO3, which is the product of the above reaction, contacts with electrolyte and cools rapidly. During this rapid cooling process, amorphous phases are formed, and some high temperature phases of BaTiO3 are reserved, such as hexagonal phase and cubic phase, (Fig.2(a)). During MAO treatment, BaTiO3 film grows layer by layer. The micro arc oxidation process model and temperature distribution are schemed in Fig.5. Different zones in the film suffer a temperature gradient. Because of heat transfer, from zone C to zone A in Fig.5, temperature reduces. Effect of mode of MAO treatment (AC or DC) on microstructure of film can be responsible for sparking duration and spacious distribution. When the spark occurs at the interface between oxide surface and electrolyte, heat transfers to the bottom layer of oxide. Indeed, the previous coating layer is heat-treated by the heat transferred from surface sparking during MAO. The longer the hold time of sparking at site C, the higher the temperature at sites B and A.

Fig.5 Model of processing of micro-arc oxidation
For AC mode, sparking is discrete and usually spaciously uniform. The temperature gradient(TG), from C to A in Fig.5 is great. Contrast to AC mode, continuous sparking occurs at some coating surface site (for instance, site C) for DC MAO. Longer duration of sparking at site C not only produces rough surface (Fig.3(a)), but also results in higher temperature in the next layer (zone B). Higher temperature at site A is beneficial to promoting microstructural changes, such as crystallization, grain growth and phase transformation. The annealing crystallization temperature of anamorphous phase of BaTiO3 is more than 650 ℃[20]. Annealing tempera- tures in this study are in the range of 900-1 200 ℃, so crystallization of the amorphous phases occurs during annealing (Fig.1(c)). Few amorphous phases in the film prepared by DC MAO (Fig.3(b) indicate that the transient temperatures at A and B are high enough to make amorphous phases crystallized, which is responsible for longer duration of sparking.
For films and fine particles of BaTiO3, as far as the transformation of cubic to tetragonal phase is concerned, Ginzburg-Landau theory is applied to establish the effect of size and surface energy on BaTiO3 transformation [17-21]. With the decrease of film thickness and particle size, Curie temperature (TC) decreases. This means that the cubic phase of BaTiO3 can be stabilized at lower temperature. This model can interpret why high temperature more than 1 000 ℃ is required to obtain tetragonal BaTiO3 during sintering[4-7]. By means of AC MAO, the grain size is less than 1 mm even after being annealed at 1 200 ℃ for 8 h (Fig.3(b)). It is plausible to suggest that the grain size in the film prepared by DC MAO is larger than that prepared by AC MAO.
During dynamic transformation of BaTiO3, the inner stress constraint is another significant factor to be taken into account. The ferroelectric transformation is sensitive to hydrostatic stress[18]. During MAO, large amount of oxygen with estimated pressure of tens of MPa forms on the anode surface[22]. In addition, the inner thermal stress exists in the coating due to the thermal misfit between the ceramic coating and substrate. The larger the stress in coating, the lower the Curie temperature TC, and the more stable the paraelectric phase. During DC MAO, higher average temperature in the film is beneficial to relaxing the thermal misfit stress.
5 Conclusions1) In 0.2 mol/L Ba(OH)2 electrolyte, with current density of 5 A/cm2, BaTiO3 films prepared by AC MAO are mainly made of amorphous phase and primitive cubic phase.
2) After being annealed at 900-1 000 ℃ for 8 h, amorphous phase in the film prepared by AC MAO can be crystallized fully. A few tetragonal phases are obtained even after being annealed at 1 200 ℃ for 8 h.
3) In 0.5 mol/L Ba(OH)2 electrolyte, with current density of 150 mA/cm2, almost absence of amorphous phase in BaTiO3 films prepared by DC MAO was observed. In these films, the constituent phases are cubic and tetragonal.
Reference
[1] ZIMMERMANN F, VOIGTS M, MENESKLOU W, IVERS- TIFFEE E. Ba0.6Sr0.4TiO3 and BaZr0.3Ti0.7O3 thick films as tunable microwave dielectrics [J]. J Euro Ceram Soc, 2004, 24: 1729-1733.
[2] ZIMMERMANN F, VOIGTS M, WEIL C, JAKOBY R, WANG P, MENESKLOU W, IVERS-TIFFEE E. Investigation of barium strontium titanate thick films for tunable phase shifters [J]. J Euro Ceram Soc, 2001, 21: 2019-2023.
[3] KAMEL F, GONON P, JOMNI F. Electrical properties of low temperature deposited amorphous barium titanate thin films as dielectrics for integrated capacitors [J]. Thin Solid Films, 2006, 504: 201-204.
[4] KAWASAKI T, KANAZAWA S, OHKUBO T, MIZERACZYK J, NOMOTO Y. Dependence of sintering temperatures of the BaTiO3 pellets on N2O generation characteristics in a packed-bed plasma reactor [J]. Thin Solid Films, 2001, 386: 177-182.
[5] CHEN R, CUI A, WANG X, GUI Z, LI L. Structure, sintering behavior and dielectric properties of silica-coated BaTiO3 [J]. Mate Lett, 2002, 54: 314-317.
[6] ZHAO J, WANG X, CHEN R, LI L. Synthesis of thin films of barium titanate and barium strontium titanate nanotubes on titanium substrates [J]. Mate Lett, 2005, 59: 2329-2332.
[7] CHEN K Y, CHEN Y W. Preparation of barium titanate ultrafine particles from rutile titania by a hydrothermal conversion [J]. Powder Tech, 2004, 141: 69-74.
[8] LICHERI R, FADDA S, ORRU R, CAO G, BUSCAGLIA V. Self-propagating high-temperature synthesis of barium titanate and subsequent densification by spark plasma sintering (SPS) [J]. J Euro Ceram Soc, 2007, 27: 2245-2553.
[9] LUO S, TANG Z, YAO W, ZHANG Z, LI J, NIAN J. Low temperature combustion synthesis and its dielectric property of barium titanate [J]. J Chinese Ceram Soc, 2003, 31(6): 560-565.
[10] XU J, ZHAI J, YAO X. Structure and dielectric properties of barium titanate thin films grown by sol-gel-hydrothermal process [J]. Appl Phys Lett, 2006, 89: 1-3.
[11] HUANG L, CHEN Z, WILSON J D, BANERJEE S, ROBINSON R D, HERMAN I P, LAIBOWEITZ R, O’BRIEN S. Barium titanate nanocrystals and nanocrystal thin films: Synthesis, ferroelectricity, and dielectric properties [J]. J Appl Phys, 2006, 100: 1-9.
[12] SCHREKENBACH J, SCHLOTTIG F, MARX G. Preparation and microstructure characterization of anodic spark deposited barium titanate conversion [J]. J Mater Res, 1999, 14: 1437-1438.
[13] WU C, LU F. Corrosion resistance of BaTiO3 films prepared by plasma electrolytic oxidation [J]. Surf Coat Tech, 2002, 166: 31-36.
[14] WU C, LU F. Synthesis of barium titanate films by plasma electrolytic oxidation at room electrolyte temperature [J]. Surf Coat Tech, 2005, 199: 225-230.
[15] LI W, HAN B, PENG J, DU J, GAO Y. Structural characteristics of BaTiO3 films prepared by microarc oxidation [J]. Trans Nonferrous Metal Soc China, 2006, 16: 1041-1044.
[16] PENG J, HAN B, LI W, GUO P, HAN D. Study on the microstructural evolution of BaTiO3 on titanium substrate during MAO [J]. Mater Letter, 2008, 62: 1801-1804.
[17] WANG B, WOO C H. Curie temperature and critical thickness of ferroelectric thin films [J]. J Appl Phys, 2005, 97: 1-9.
[18] LI W, WENG G J. Micromechanics simulation of spontaneous polarization in ferroelectric crystals [J]. J Appl Phys, 2001, 90(5): 2484-2491.
[19] KHASELEV O, WEISS D, YAHALOM J. Structure and composition of anodic films formed on binary Mg-Al alloys in KOH-aluminate solutions under continuous sparking [J]. Corr Sci, 2001, 43: 1295- 1307.
[20] THOMAS R, DUBE D C, KAMALASANAN M N. Optical and electrical properties of BaTiO3 thin films prepared by chemical solution deposition [J]. Thin Solid Films, 1999, 346: 212-225.
[21] UCHINO K, SADANAGE E, HIROSE T. Dependence of the crystal structure on particle size in barium titanate [J]. J Am Ceram Soc, 1989, 72: 1555-1558.
[22] SNIZHKO L O, YEROKHIN A L, PILKINGTON A, GUREVINA N L, MISNIYANKIN D O, LEYLAND A, MATTHEWS A. Anodic processes in plasma electrolytic oxidation of aluminium in alkaline solutions [J]. Electrochimica Acta , 2004, 49: 2085-2095.
Foundation item: Project(51412020203JW1609) supported by the Advanced Research Foundation of Weapon Equipment, China
Corresponding author: PENG Ji-hua; Tel: +86-20-87113747; E-mail: jhpeng@scut.edu.cn
Abstract: BaTiO3 films on Ti substrate were fabricated by alternative current(AC) and direct current(DC) micro arc oxidation (MAO). Microstructures of films were investigated by means of SEM, XRD and TEM. The results show that the amorphous phase and primitive cubic phase are the main phases in the films prepared by AC MAO. Even after being annealed at 1 200 ℃ for 8 h, only a few tetragonal phases can be observed in films prepared by AC MAO. However, tetragonal BaTiO3 phase can be produced by DC MAO directly. In the films prepared by DC MAO, a mixture of cubic phase and tetragonal phase is formed. After sparking spacious distribution, sparking duration and temperature gradient near sparking sites were taken into account, and a mechanism of synthesis of tetragonal BaTiO3 phase by DC MAO was proposed.


