Trans. Nonferrous Met. Soc. China 25(2015) 647-653
Copper adsorption by natural manganese dioxide
Nizamettin 
Department of Chemical Engineering, Faculty of Engineering, Inonu University, Malatya 44280, Turkey
Received 8 January 2014; accepted 19 May 2014
Abstract:
The adsorption of copper ions was investigated using pyrolusite ore as a low-cost alternative adsorbent source. Pyrolusite, which contains mainly MnO2, is a manganese ore. The effects of the initial concentration of copper(II) ions, initial pH of solution, adsorbent dosage and particle size on the adsorption process were examined. It was found that the percentage of the adsorbed copper increases with increasing the amount of adsorbent. It was observed that the maximum adsorption occurred at natural initial pH values for all copper concentrations. While the initial solution concentration, initial pH, contact time, stirring speed, particle size and adsorbent dosage were 2.5 mmol/L, natural, 180 min, 200 r/min, 120 μm and 6 g/L, respectively, the efficiency of copper adsorption on pyrolusite ore was 96.5%. The isotherm and kinetic studies relating to this adsorption process were also made. It was determined that the equilibrium data followed the Langmuir isotherm model while the process kinetic could be described by the pseudo-second order kinetic model.
Key words:
adsorption; copper; adsorbent; pyrolusite ore;
1 Introduction
The resulting effluents at the end of many industrial processes can contain some heavy metals, such as Cd, Cr, Cu, Co, Pb and Zn. Industrial effluents including heavy metals are generally discharged into environment. The discharged wastewater can induce serious damages on the environment and human health due to its metal content. Therefore, heavy metals in industrial wastewater should be removed using appropriate separation methods [1-4]. Because it cannot be destroyed chemically like organic pollutants, several treatment methods have been developed to remove heavy metals ions from wastewater [5,6]. For the recovery or removal of metal ions from aqueous solution, many techniques, such as chemical precipitation, ultra-filtration, reverse osmosis, electrodeposition, solvent extraction, filtration, ion exchange, cementation and adsorption, can be used [1,4,5,7,8]. When they are compared with each other, each of these methods has some advantages and disadvantages. Thus, the most effective technique or techniques should be applied to remove heavy metals from aqueous solution.
Among the methods mentioned above, adsorption process is one of the most widely used methods for the removal of metal ions from an aqueous solution. This method is especially more suitable for the removal of the metal ions from diluted solution. Activated carbon obtained from various sources is often used as adsorbent for the removal of metal ions due to its high adsorption capacity. However, activated carbon is not economically feasible for large scale applications because of its high cost of production and regeneration [3,9,10]. Hence, the use of the adsorbents with low cost can be advantageous in adsorption process. The materials that have been used for this purpose include both natural materials and wastes, and byproducts generated from many industries. These materials are widely known as low-cost adsorbents [11-13]. Among these alternative adsorbent sources, natural materials (ores or minerals) can be used for the removal of various species from wastewater owing to its low cost and abundance.
Manganese is the twelfth most abundant element in the shell of the earth. The most common mineral is pyrolusite, which is mainly MnO2. Low-grade pyrolusite ore in its natural state has little commercial value. For this reason, it is usually treated and converted into the metal or other more valuable manganese compounds. The ore is essentially used for the production of manganese sulfate [14-16]. The ore contains iron oxide, silica, and clay minerals in addition to manganese oxide depending on the ore source. These oxides and clay minerals in ore matrix have adsorbent feature, and they exhibit high potential for heavy metal adsorption. Hence, pyrolusite ore can be used as a low-cost adsorbent for the adsorption of metal ions from wastewater because of its abundance in the natural state, ease of availability and direct use without pretreatment [17-20]. The adsorption of various inorganic and organic species using pyrolusite and manganese nodules has been examined. A low-cost ferruginous manganese ore was used by CHAKRAVARTY et al [17] for the removal of arsenic from groundwater. They found that almost 100% arsenic was removed. ROUT et al [18] examined the adsorption behavior of Pb(II), Cd(II) and Zn(II) ions on a low-grade manganese ore. They investigated the effects of contact time, pH, adsorbent and adsorbate concentration, and temperature on adsorption process. They determined that manganese ore had high loading capacity for the indicated ions. AJMAL et al [20] studied the adsorption of Pb2+, Zn2+ and Mg2+ from aqueous solutions using pyrolusite. They suggested that pyrolusite might provide an economical method for removal metal ions from industrial wastewaters. BERNARD et al [21] studied the adsorption ability of various organic micropollutants by pyrolusite. Manganese nodule residue obtained from NH3-SO2 leaching was used for copper adsorption [22]. The loading capacity of the leach residue was found to be higher than that of activated charcoal. It was determined that the adsorption of copper ions followed the Langmuir isotherm, and the maximum adsorption occurred at pH between 4 and 5.  et al [23] examined the adsorption of lead and cadmium ions from aqueous solution using manganese oxide minerals as the low cost adsorbent.
et al [23] examined the adsorption of lead and cadmium ions from aqueous solution using manganese oxide minerals as the low cost adsorbent.
The aim of this study is to examine the use of pyrolusite as an alternative adsorbent material for the removal of copper (II) ions from aqueous solution. The effects of the experimental parameters, including initial solution concentration, adsorbent dosage, initial solution pH and particle size on the adsorption of copper (II) ions from aqueous solution were examined. In addition, the kinetic and isotherm studies were also performed using experimental data.
2 Experimental
2.1 Materials
The pyrolusite ore sample used in this study was provided from Konya, Turkey. The ore sample was crushed, ground, and then sieved using standard test sieves to obtain different particle size fractions. The original ore sample was analyzed, and it was determined that the mineral contained 57.66% MnO2, 10.00% CaO, 8.04% Fe2O3, 7.08% SiO2, 0.93% Al2O3, 0.44% MgO, 15.85% loss of ignition at 800 °C. The mineralogical composition of the pyrolusite ore was determined by X-ray diffraction analysis using a Rigaku RadB-DMAX II model X-ray diffractometer. The X-ray diffraction pattern of the ore sample is shown in Fig. 1. This figure indicates that the ore sample used was mainly composed of pyrolusite (MnO2), phyllosilicate, quartz and calcite.
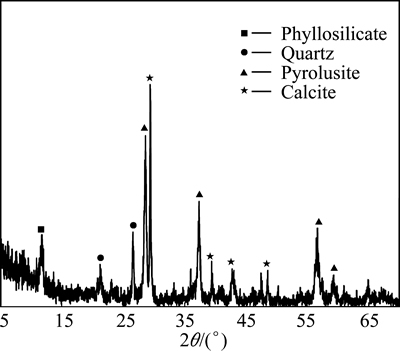
Fig. 1 X-ray diffraction pattern of ore sample
All the chemicals used in the adsorption experiments were analytical grade and were employed as received without further purification. Chemical substances used in this study included CuSO4·5H2O, H2SO4, Titriplex III and murexide. Distilled water was used to prepare all solutions.
2.2 Adsorption tests
The solutions containing Cu2+ ions were prepared by dissolving weighed quantities of CuSO4·5H2O in distilled water. The pH values of the solutions were determined by WTW pM×2000 pH/ION meter. The initial pH of sample solutions was adjusted using diluted H2SO4. All adsorption experiments were carried out at laboratory temperature (23±1 °C). The batch adsorption tests were performed in 100 mL conical flasks placed on a magnetic stirrer. In each adsorption test, after the volume of 50 mL copper solution at a definite concentration was placed into the glass flask, a given amount of adsorbent was added to the solution. The process was performed for various contact time. At the end of each contact time, the content of the flask was filtered using filter paper, and the amount of copper ions in the solution was determined complexometrically using Titriplex III solution (0.01 mol/L) as titrant and murexide as indicator. The amount of copper adsorbed by pyrolusite ore was calculated from the difference between the metal ion concentration before and after adsorption process. The percentage of copper removal and the amount of copper adsorbed per unit mass of ore were evaluated using the equations as follows:
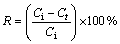 (1)
(1)
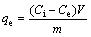 (2)
(2)
where R is the removal rate; Ci and Ct are the initial concentration and residual concentration for t min, respectively (mg/L); Ce is the equilibrium concentration (mg/L); qe is the amount of adsorbed ion per unit mass of adsorbent at equilibrium (mg/g); V is the initial volume of the adsorption solution (L); m is the mass of adsorbent (g).
3 Results and discussion
3.1 Determination of optimal adsorbent dosage
To determine the optimum adsorbent dosage for different concentrations of copper ions, the experiments were carried out at four different pyrolusite dosages in the range of 0.2-0.5 g while the initial concentrations of the solution containing copper ions were changed from 1.0 to 7.5 mmol/L. While these tests were performed, the values of the contact time, total solution volume, particle size and agitation speed of solution were 24 h, 50 mL, 120 μm and 200 r/min, respectively. In these experiments, the initial pH of copper solutions was natural pH value for relevant concentration (see Table 1). Figure 2 shows the effect of adsorbent dosage on the adsorption of copper ions at different initial concentrations. As shown in Fig. 2, the percentage of the adsorbed copper ions decreased for the same amount of pyrolusite as the initial concentration increased. In other words, the amount of the adsorbed copper increased for the same initial concentration of copper ions when the amount of adsorbent increased. After being contacted for 24 h, the extent of removal of copper from the solution at the amount of adsorbent dosage of 0.3 g were 99.5%, 96.5% and 94%, respectively, whereas for the same contact time and adsorbent dosage, only 60% copper was adsorbed by pyrolusite ore with the initial concentration of 7.5 mmol/L. An increase of the adsorbent dosage for a given initial ion concentration or a decrease of the adsorbate concentration for a defined adsorbent dosage provides a greater surface area or adsorption sites between pyrolusite and copper ion. Thus, the extent of copper adsorption increases. From the experimental data obtained, the optimal adsorbent dosage was selected to be 0.3 g in the subsequent tests.
Table 1 pH values of copper sulfate solutions at different concentrations

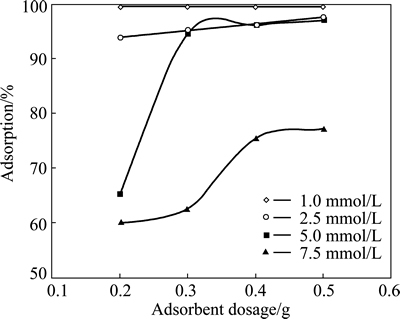
Fig. 2 Effect of adsorbent dosage on copper adsorption
3.2 Determination of optimal initial pH
The effect of initial pH of solution on copper adsorption was investigated at pH value of 3, 4 and natural values. In these tests, the initial concentration of copper ions was varied in the range of 2.5-7.5 mmol/L, while the contact time, total volume, stirring speed, particle size and adsorbent dosage were fixed at 24 h, 50 mL, 200 r/min, 120 μm and 0.3 g, respectively. The results obtained from these experiments are shown in Fig. 3. It can be seen from the figure that the extent of copper adsorption increased for all copper concentrations with increasing the initial pH value. The maximum extent of adsorption was reached at natural pH for all initial copper concentrations. When the initial pH of the solution was lower than 3, pyrolusite ore used as adsorbent may dissolve. As listed in Table 1, natural pH values of copper solutions used in this study varied in the range of 5.52-5.10. In the range of pH values, the precipitation of copper species was not observed.
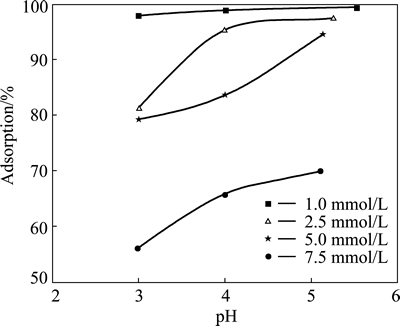
Fig. 3 Effect of initial pH on copper adsorption
However, the formation of a copper precipitate may occur at higher pH values than at natural pH of solutions. Furthermore, the adsorbent surface may be protonated when pH<3. In this situation, the repulsion of copper ions from adsorbent surface may occur. Therefore, the extent of adsorption may decrease. Because of these reasons mentioned above, the experiments were not performed at lower and higher pH values.
The effect of pH on adsorption was also examined for various contact time. Figure 4 shows the results of these experiments. As shown in Fig. 4, the percentage of the adsorbed copper increased with increasing pH value. In these tests, the initial concentration of the solution containing copper (II) ions, initial pH value, particle size, adsorbent dosage, total volume and stirring speed were 2.5 mmol/L, natural, 120 μm, 0.3 g, 50 mL and 200 r/min, respectively.
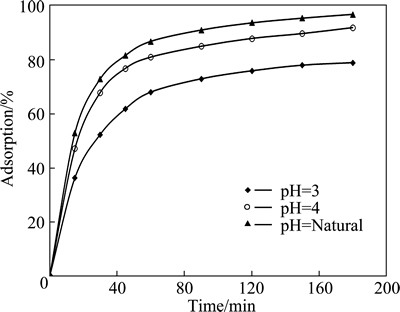
Fig. 4 Effect of initial pH on copper adsorption for various contact time
3.3 Effect of initial concentration on adsorption
The effect of initial copper concentration on adsorption was studied at the concentrations of 1.0, 2.5, 5.0 and 7.5 mmol/L. In these tests, the values of the initial pH of solution, total volume, adsorbent dosage, particle size and stirring speed were selected to be natural, 50 mL, 0.3 g, 120 μm and 200 r/min, respectively. The results obtained from these experiments are shown in Fig. 5. This figure shows the extent of adsorption decreased with increasing the initial copper concentration for the same adsorbent dosage. This result indicates that the initial concentration of copper has a major effect on adsorption process.
3.4 Effect of particle size of adsorbent on adsorption
Since the adsorption extent is proportional to the surface area of the solid adsorbent, more finely divided solid can provide more surface area per unit mass of the solid adsorbent. Hence, the effect of particle size on copper adsorption was investigated using the average size of 160, 140, 120 and 93 μm. In these experiments, the values of the initial concentration of solution, total volume, initial pH, adsorbent dosage, and stirring speed were selected to be 2.5 mmol/L, 50 mL, natural, 0.3 g and 200 r/min, respectively. The results obtained from these experiments are shown in Fig. 6. As shown in Fig. 6, the percentage of the adsorbed copper increased with decreasing the particle size of the adsorbent.
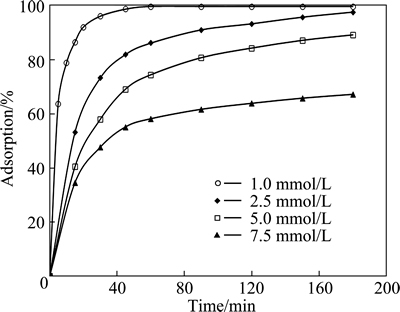
Fig. 5 Effect of initial concentration on copper adsorption for various contact time
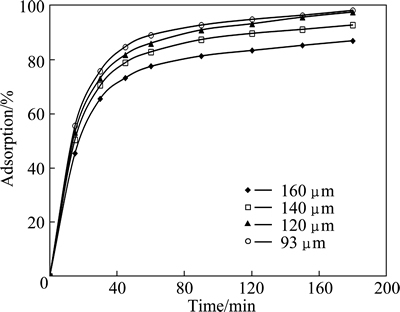
Fig. 6 Effect of particle size on copper adsorption for various contact time
3.5 Adsorption isotherms
In adsorption process, the equilibrium between the adsorbent and adsorbate is described by adsorption isotherms. The isotherms show the relationship between the equilibrium concentration in the solid phase and in the aqueous phase for the adsorbate molecules when the process reaches an equilibrium state. Various adsorption isotherms have been developed to realize the interaction between the adsorbed molecules and the solid adsorbent. Among these isotherm models, the Freundlich and Langmuir models are the most widely applied isotherms for various adsorption processes [24,25].
In this study, these two isotherm models were applied to analyze the adsorption data obtained from the experiments. The Freundlich model assumes that the adsorption occurs on the heterogeneous surface of the solid adsorbent. On the other hand, the Langmuir model assumes that the adsorption takes place on a homogeneous surface by monolayer sorption without interaction between the adsorbed molecules [26,27]. The linear forms of the Freundlich and Langmuir isotherm equations are given as follows:
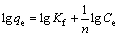 (Freundlich model) (3)
(Freundlich model) (3)
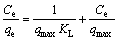 (Langmuir model) (4)
(Langmuir model) (4)
where qe is the amount of adsorbate adsorbed per unit mass of adsorbent (mg/g); Ce is the equilibrium concentration of the adsorbate (mg/L); Kf is the Freundlich constants, including the adsorption capacity of the adsorbent (L/g); n is the adsorption intensity of adsorbent; qmax is the monolayer adsorption capacity of the adsorbent (mg/g); KL is the Langmuir constant (L/g).
If the adsorption process obeys the Freundlich model, then the left side (lg qe) of Eq. (3) versus lg Ce must be straight line relationship. Figure 7 shows the plot of lg qe versus lg Ce for various concentrations. On the other hand, if the adsorption process obeys the Langmuir model, then the left side (Ce/qe) of Eq. (4) versus Ce must be straight line relationship. Figure 8 shows the plot of Ce/qe versus Ce for various concentrations. As shown in Fig. 7 and Fig. 8, the straight lines were formed for each two models. In Eq. (3), the slope of the lines is n-1, and the intercept is lg Kf. In Eq. (4), the slope of the straight lines is qmax-1, and the intercept is qmax-1·KL. The values of these parameters determined from Figs. 7 and 8 are listed in Table 2.
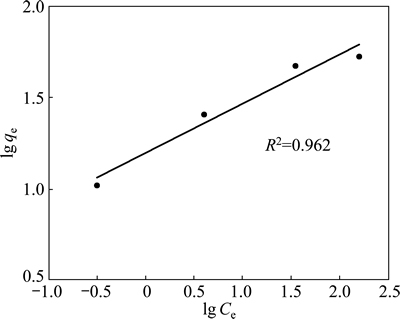
Fig. 7 Freundlich isotherm plot for copper adsorption on pyrolusite ore
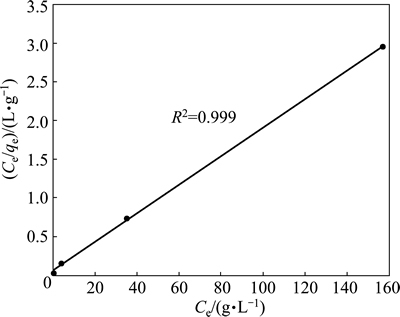
Fig. 8 Langmuir isotherm plot for copper adsorption on pyrolusite ore
Table 2 Langmuir and Freundlich isotherm parameters and correlation coefficients for copper adsorption on pyrolusite ore

When the regression coefficients are evaluated in Table 2, it can be seen that bigger regression coefficients were found for the Langmuir model. In addition to this information, it is expressed that the main properties of the Langmuir isotherm can be described by a dimensionless constant called equilibrium parameter or separation factor [28]. This is expressed as follows:
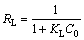 (5)
(5)
where RL is the dimensionless constant called separation factor or equilibrium parameter; KL is the Langmuir constant (L/mg); C0 is the initial concentration of solution (mg/L). The values of RL indicate the nature of the adsorption process. A value of RL in the range from 0 to 1 indicates that the adsorption process between the adsorbent and adsorbate is favorable under the experimental conditions performed. This also indicates that the Langmuir isotherm is valid for adsorption process. The values of RL were found to be 0.053, 0.022, 0.011, and 0.007 at the copper concentrations of 1.0, 2.5, 5.0 and 7.5 mmol/L, respectively. All the findings mentioned above show that the adsorption of copper by pyrolusite ore follows the Langmuir isotherm model.
3.6 Adsorption kinetics
The kinetic analysis of the adsorption processes is generally performed using pseudo-first order and pseudo-second order kinetic models [29]. The equations describing these kinetics models are given as follows:
ln(qe-qt)=ln qe-k1t(Pseudo-first order kinetic) (6)
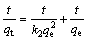 (Pseudo-second order kinetic) (7)
(Pseudo-second order kinetic) (7)
where qe is the amount of the adsorbed metal ion at equilibrium (mg/g); qt is the amount of the adsorbed metal ion for t min (mg/g); k1 is the rate constant for the first order adsorption (1/min); k2 is the rate constant for the second order adsorption (g·mg-1·min-1); t is the adsorption time (min).
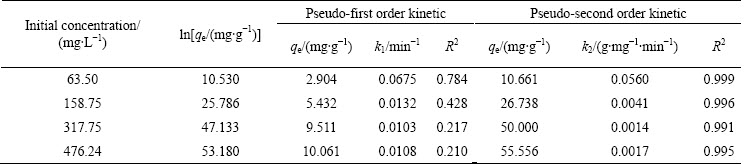
To determine the kinetic model of the adsorption between pyrolusite ore and copper ions, the plot of ln(qe-qt) versus t for the pseudo-first order kinetic was drawn using the experimental data obtained. The slope of the straight line obtained is k1, and the intercept is ln qe. For the pseudo-second order kinetic, the plot of the left side of Eq. (7), t/qe, versus t is constructed. The slope of the straight line is 1/qe, and the intercept is k2-1qe2. It was observed that the low regression coefficients were obtained for the pseudo-first order kinetic model. Figure 9 shows the graph constructed for pseudo-second order kinetic model. The values of the rate constants and regression coefficients determined from Fig. 9 are listed in Table 3. The highest regression coefficients were obtained for the pseudo-second order kinetic. Also, it can be seen in Table 3 that the qe values calculated from the intercepts of the straight lines in Fig. 9 are in good agreement with the qe values determined experimentally. The values of the regression coefficients and the agreement between the qe values indicate that this adsorption process follows the pseudo-second order kinetic.
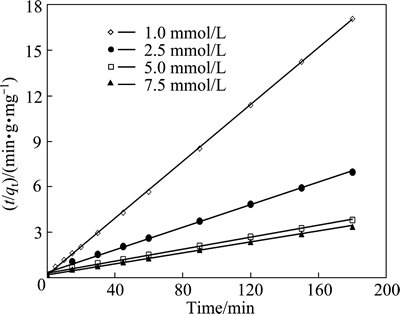
Fig. 9 Pseudo-second order kinetic plot for copper adsorption
4 Conclusions
1) Natural manganese dioxide, which is known as pyrolusite ore, was used as an alternative adsorbent for the removal of copper(II) ions from wastewaters.
2) The effects of the initial concentration of solution, adsorbent dosage, particle size of pyrolusite ore and initial pH of solution on the copper adsorption were examined. It was shown that the percentage of the adsorbed copper increased with increasing the adsorbent dosage and initial pH, and with decreasing the particle size and initial concentration. It was observed that the maximum adsorption occurred at natural initial pH values for all copper concentrations. According to the experimental conditions, it was found that almost all of copper ions were removed from aqueous solution. While the initial solution concentration, initial pH, contact time, stirring speed, particle size and adsorbent dosage were 2.5 mmol/L, natural, 180 min, 200 r/min, 120 μm and 6 g/L, respectively, the efficiency of copper adsorption on pyrolusite ore was 96.5%.
3) The isotherm and kinetic studies show that the equilibrium data follow the Langmuir isotherm model while the process kinetic obeys the pseudo-second order kinetic model. According to these data obtained, it can be included that the copper adsorption process by pyrolusite ore occurs by chemisorption. The present work suggests that the pyrolusite ore (natural manganese dioxide) can be used as a relatively low-cost adsorbent for the removal of metal ions from wastewater because the ore is available in its natural state without any need for the preliminary process.
Table 3 Kinetic parameters for adsorption of copper ions on pyrolusite ore
References
[1] LIU D, SUN D, LI Y. Removal of Cu(II) and Cd(II) from aqueous solutions by polyaniline on sawdust [J]. Sep Sci Technol, 2011, 46: 321-329.
[2] DELIYANNI E A, LAZARIDIS N K, PELEKA E N, MATIS K A. Metal removal from aqueous solution by iron-based bonding agents [J]. Environ Sci Pollut Res, 2004, 11: 18-21.
[3] REN T, HE P, NIU W, WU Y, AI L, GOU X. Synthesis of α-Fe2O3 nanofibres for applications in removal and recovery of Cr(VI) from wastewater [J]. Environ Sci Pollut Res, 2013, 20(1): 155-162.
[4] NGUYEN N V, JEONG J, LEE J. Removal of chromium (VI) from the leachate of electronic scrap using non-ionic Amberlite XAD-7HP resin [J]. J Chem Technol Biotechnol, 2013, 88(6): 1014-1022.
[5] 
 . Heavy metal removal form aquatic systems by Northern Anatolian smectites [J]. Turk J Chem, 2000, 24: 209-215.
. Heavy metal removal form aquatic systems by Northern Anatolian smectites [J]. Turk J Chem, 2000, 24: 209-215.
[6] FONSECA M G, OLIVERIA M M, ARAKAKI L N H. Removal of cadmium, zinc, manganese and chromium cations from aqueous solution by a clay mineral [J]. J Hazard Mater, 2006, 137: 288-292.
[7] OKIEIMEN F E, SOGBAIKE C E, EBHOAYE J E. Removal of cadmium and copper ions from aqueous solution with cellulose graft copolymers [J]. Sep Sci Technol, 2005, 44: 85-89.
[8] DEMIRKIRAN N,  A. Recovering of copper with metallic aluminum [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 2778-2782.
A. Recovering of copper with metallic aluminum [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 2778-2782.
[9] DAVE P N, SUBRAHMANYAM N, SHARMA S. Kinetics and thermodynamics of copper ions removal from aqueous solution by use of activated charcoal [J]. Ind J Chem Technol, 2009, 16: 234-239.
[10] BELLO O S, ADELAIDE O M, HAMMED M A, POPOOLA O A M. Kinetic and equilibrium studies of methylene blue removal from aqueous solution by adsorption on treated sawdust [J]. Maced J Chem Chem Eng, 2010, 29: 77-85.
[11] AGRAWAL A, SAHU K K, PANDEY B D. A comparative adsorption study of copper on various industrial solid wastes [J]. AIChE J, 2004, 50: 2430-2438.
[12] KURNIAWAN T A, CHAN G Y S, LO W H, BABEL S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals [J]. Sci Total Environ, 2006, 366: 409-426.
[13] GUPTA V K, CARROTT P J M, RIBEIRO CARROTT M M L, SUHAS. Low-cost adsorbents: growing approach to wastewater treatment—A review [J]. Critic Rev Environ Sci Technol, 2009, 39: 783-842.
[14] ZHANG W, CHENG C Y. Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide [J]. Hydrometallurgy, 2007, 89: 137-159.
[15] NAYL A A, ISMAIL I M, ALY H F. Recovery of pure MnSO4·H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide [J]. Int J Miner Process, 2011, 100: 116-123.
[16] EKMEKYAPAR A,  BAYSAR A, CEYLAN K. Reductive leaching of pyrolusite ore by using sawdust for production of manganese sulfate [J]. Russ J Non-Ferrous Met, 2012, 53: 211-217.
BAYSAR A, CEYLAN K. Reductive leaching of pyrolusite ore by using sawdust for production of manganese sulfate [J]. Russ J Non-Ferrous Met, 2012, 53: 211-217.
[17] CHAKRAVARTY S, DUREJA V, BHATTACHARYYA G, MAITY S, BHATTACHARJEE S. Removal of arsenic from groundwater using low cost ferruginous manganese ore [J]. Water Res, 2002, 36: 625-632.
[18] ROUT K, MOHAPATRA M, MOHAPATRA B K, ANAND S. Pb(II), Cd(II) and Zn(II) adsorption on low grade manganese ore [J]. Int J Eng Sci Technol, 2009, 1: 106-122.
[19] LIU C, WANG X, LI X, CAO W, YANG J. Detoxification of arsenite through adsorption and oxidative transformation on pyrolusite [J]. Clean-Soil Air Water, 2012, 40: 1265-1272.
[20] AJMAL M, RAO R A K, SIDDIQUI B A. Adsorption studies and the removal of dissolved metals using pyrolusite as adsorbent [J]. Environ Monitor Assess, 1995, 38: 25-35.
[21] BERNARD S, CHAZAL P H, MAZET M. Removal of organic compounds by adsorption on pyrolusite (β-MnO2) [J]. Water Res, 1997, 13: 1216-1222.
[22] SHAOO R N, DAS S C, REDDY B R, RATH P C, DAS R P. Adsorption of copper on manganese nodule residue obtained from NH3-SO2 leaching [J]. Hydrometallurgy, 2001, 62: 185-192.
[23]  Adsorption of lead and cadmium ions from aqueous solutions using manganoxide minerals [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 3131-3139.
Adsorption of lead and cadmium ions from aqueous solutions using manganoxide minerals [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 3131-3139.
[24] TAN I A W, HAMEED B H, AHMAD A L. Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon [J]. Chem Eng J, 2007, 127: 111-119.
[25] HAMEED B H, AHMAD A A. Batch adsorption of methylene blue aqueous solution by garlic peel, an agricultural waste biomass [J]. J Hazard Mater, 2009, 164: 870-875.
[26] WANG X S, HUANG J, HU H Q, WANG J, QIN Y. Determination of kinetic and equilibrium parameters of the batch adsorption of Ni(II) from aqueous solutions by Na-mordenite [J]. J Hazard Mater, 2007, 142: 468-476.
[27] CHANG Y Y, LIM J W, YANG J K. Removal of As(V) and Cr(VI) in aqueous solution by sand media simultaneously coated with Fe and Mn oxides [J]. J Ind Eng Chem, 2012, 18: 188-192.
[28] KAVITHA D, NAMASIVAYAM C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon [J]. Bioresour Technol, 2007, 98: 14-21.
[29] WANG L H, LIN C, WU F C. Kinetic study of adsorption of copper (II) ion from solution using rice hull ash [J]. J Taiwan Inst Chem Eng, 2010, 41: 599-605.
利用天然二氧化锰吸附铜
Nizamettin 
Department of Chemical Engineering, Faculty of Engineering, Inonu University, Malatya 44280, Turkey
摘 要:软锰矿的主要成分为MuO2,其可作为一种低成本的吸附剂使用,研究其对废水中铜离子的吸附分离作用。研究Cu(II)离子的初始浓度、溶液初始pH值、吸附剂用量和粒度对吸附过程的影响。结果表明:随着吸附剂的用量增加,吸附铜的比例增大。在不同铜浓度下,溶液的初始pH值为自然状态时的吸附量最大。当初始溶液浓度、初始pH值、接触时间、搅拌速度、粒径大小和吸附剂用量分别为0.0025 mol/L、自然状态、180 min、200 r/min和6 g/L时,软锰矿对铜的吸附率为96.5%。对吸附过程中的等温吸附曲线和动力学进行研究。结果表明:该平衡吸附数据符合Langmuir等温模型,而过程的动力学符合伪二阶动力学模型。
关键词:吸附;铜;吸附剂;软锰矿
(Edited by Chao WANG)
Corresponding author: Nizamettin  ; Tel: +90-4223774760; E-mail: nizamettin.demirkiran@inonu.edu.tr
; Tel: +90-4223774760; E-mail: nizamettin.demirkiran@inonu.edu.tr
DOI: 10.1016/S1003-6326(15)63648-2
Abstract: The adsorption of copper ions was investigated using pyrolusite ore as a low-cost alternative adsorbent source. Pyrolusite, which contains mainly MnO2, is a manganese ore. The effects of the initial concentration of copper(II) ions, initial pH of solution, adsorbent dosage and particle size on the adsorption process were examined. It was found that the percentage of the adsorbed copper increases with increasing the amount of adsorbent. It was observed that the maximum adsorption occurred at natural initial pH values for all copper concentrations. While the initial solution concentration, initial pH, contact time, stirring speed, particle size and adsorbent dosage were 2.5 mmol/L, natural, 180 min, 200 r/min, 120 μm and 6 g/L, respectively, the efficiency of copper adsorption on pyrolusite ore was 96.5%. The isotherm and kinetic studies relating to this adsorption process were also made. It was determined that the equilibrium data followed the Langmuir isotherm model while the process kinetic could be described by the pseudo-second order kinetic model.


