非那雄胺/聚碳酸亚丙酯马来酸酯缓释微球的制备与性能
彭东明1, 2,张航1,王晓红1,刘艳飞1
(1. 中南大学 化学化工学院,湖南 长沙,410083;
2. 湖南中医学院 药学院,湖南 长沙,410208)
摘 要:
碳酸亚丙酯马来酸酯(PPCM)为载体,采用Oil/Water单乳液溶剂挥发法制备药物非那雄胺(finasteride)的缓释微球,并研究聚合物PPCM与药物finasteride的质量比对微球特性的影响。研究结果表明:所得PPCM微球外观圆整,平均粒径约为2 μm。随着非那雄胺比例的增加,微球的载药量提高,而药物的包封率则明显降低。在m(PPCM):m(finasteride)为5:1的条件下,获得较高的载药量和包封率,分别为14.78%和66.17%。在pH 7.4的磷酸盐缓冲溶液中,载药微球的体外释放时间达42 d,药物累积释放量为(92.59±2.62)%。微球的释药特性符合Higuchi方程Qt = 3.11+15.07 t1/2。PPCM适用于长效缓释药物传递系统。
关键词:
中图分类号:O633 文献标志码:A 文章编号:1672-7207(2014)07-2171-05
Preparation and properties of finasteride loaded poly(propylene carbonate maleate) sustained release microspheres
PENG Dongming1, 2, ZHANG Hang1, WANG Xiaohong1, LIU Yanfei1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, China)
Abstract: The novel polymer poly(propylene carbonate maleate) (PPCM) was used as drug carriers, PPCM microspheres loaded with drug finasteride were prepared by oil-in-water emulsion solvent evaporation method, and the effects of mass ratios of polymer to drug on the properties of PPCM microspheres were examined. The results show that PPCM microspheres have spherical surface, and the mean diameter of microspheres is approximately 2 μm. With the increase of finasteride proportion, the drug loading increases, while the encapsulation efficiency decreases obviously. Greater drug loading and encapsulation efficiency of microspheres are 14.78% and 66.17%, respectively when m(PPCM):m(finasteride) is 5:1. In vitro drug release of microspheres is performed in a pH 7.4 phosphate-buffered solution. The microspheres are proved to be successful in prolonging drug release up to 42 d, and cumulative drug release is (92.59±2.62)%. The properties of drug release from microspheres follow the Higuchi matrix model Qt = 3.11+15.07 t1/2. It is suggested that PPCM is suitable for long-term sustained release drug delivery system.
Key words: finasteride; poly(propylene carbonate maleate); sustained release microspheres; drug delivery systems
以可降解高分子材料为载体的药物控制释放体系是药物应用研究的热点。目前,用于缓控释载药体系的可生物降解聚合物材料主要有聚丙交酯[1-2]、聚乙交酯丙交酯[3-4]、聚己内酯[5]、聚酸酐[6]及脂肪族聚碳酸酯[7]。脂肪族聚碳酸酯是一类可生物降解、具有良好生物相容性的聚合物。作为一种很有应用前景的药物缓释材料,而受到众多研究者所关注[8]。脂肪族聚碳酸酯通过酯基[9]、羧基[10]等功能基团的修饰,可提高其热性质和降解性能。近些年来,从分子设计的角度出发,含有酯结构的新的聚碳酸酯不断被开发应用到医药领域[11-12]。Liu等[13]采用阴离子配位聚合方法,合成了二氧化碳(CO2)、环氧丙烷(PO)与马来酸酐(MA)的三元共聚物,聚碳酸亚丙酯马来酸酯(PPCM)。该三元共聚物具有良好的生物可降解性能,有望作为一种新型的药物缓释材料。非那雄胺(finasteride)是一种5-α还原酶抑制剂,干扰某些雄性激素对前列腺的影响。美国PDF批准用来治疗良性前列腺增生(BPH)、男性雄激素性脱发[14]。此外,非那雄胺也用于前列腺癌的预防[15]。该类疾病是中老年男性常见的疾病,非那雄胺是具有确切疗效的药物。然而,由于该类疾病需要较长的治疗周期,长期用药可能存在一定的副作用,而缓释制剂在维持血药浓度水平、减少副作用和提高病人的依从性方面具有明显的优势[16]。因此,非那雄胺缓控释制剂的研究具有重要的意义。单乳液 Oil/Water(O/W)溶剂挥发法适用于油溶性药物载药微球的制备[17],由于非那雄胺为油溶性药物,本文作者以PPCM为药物载体,采用单乳液 O/W溶剂挥发法来形成稳定的O/W乳液,并在分离干燥后获得PPCM-finasteride载药微球。并探讨PPCM微球制备工艺中各因素对PPCM载药性能的影响,在模拟人体生理环境条件下,进行了药物的体外释药研究。
1 实验
1.1 材料
材料有:CO2,纯度>99.5%;聚合物负载双金属型催化剂(PBM:P-Zn[Fe(CN)6]aCl2-3a(H2O)b,P为聚醚类螯合试剂,a=0~1,b=0~3);环氧丙烷,分析纯,经4 分子筛干燥后使用;非那雄胺由上海新亚药业有限公司赠送;聚乙烯醇PVA-124,醇解度98%~99%,平均聚合度为2 400~2 500;马来酸酐、磷酸二氢钾、磷酸氢二钠及其他试剂和溶剂为分析纯。
分子筛干燥后使用;非那雄胺由上海新亚药业有限公司赠送;聚乙烯醇PVA-124,醇解度98%~99%,平均聚合度为2 400~2 500;马来酸酐、磷酸二氢钾、磷酸氢二钠及其他试剂和溶剂为分析纯。
1.2 PPCM的制备
聚合物PPCM(重均相对分子质量MW=67 400,数均相对分子质量Mn=32 100,MW/Mn= 2.10)自制[13],聚合反应在300 mL 磁力搅拌高压反应釜内进行。氮气吹扫高压反应釜10 min 后,加入催化剂PBM 1.0 g、0.5 mol 环氧丙烷、0.3 mol 马来酸酐和70 mL 甲苯溶剂,室温搅拌0.5 h 后,通入CO2气体至反应体系压力增加至4 MPa 左右。加热至60 ℃,反应24 h。出料后洗涤、干燥得粗产物。再纯化得聚合物PPCM。
1.3 微球制备
以聚合物PPCM作为药物载体,采用O/W型单乳化-溶剂挥发法进行PPCM-finasteride微球的制备。制备步骤如下:称取PPCM 200 mg及一定质量的非那雄胺溶于5 mL 二氯甲烷(DCM)溶液中,在50 Hz超声场中均匀分散。将此溶液在高速搅拌下注入到100 mL 0.5% PVA水溶液中,搅拌、乳化10 min,形成稳定的O/W乳液,于室温下以700 r/min持续搅拌3 h 使DCM挥发完全,形成微球。然后将获得的微球悬浮液离心分离,并用蒸馏水洗涤2次后,冷冻干燥,得PPCM-finasteride载药微球。
1.4 微球表征
通过微球的形态、粒度、微球载药量和包封率来对微球进行表征。利用光学显微镜对制得的乳液中的微球进行观察;采用扫描电子显微镜观察微球样品形貌;粒度及分布采用激光粒度仪测定;并采用广角射线衍射对非那雄胺和非那雄胺载药微球的样品进行X衍射测定,测微球的聚集态。为了有效地测定微球的载药量和包封率,将一定量微球样品溶于二氯甲烷中,再通过HPLC法测定非那雄胺的含量。分离条件为:采用反相C18柱(Diamosil 4.6 mm×200 mm,5 μm)。检测波长为210 nm。流动相是体积比为8:2.5:1的水-乙腈-四氢呋喃溶液,流速为1.0 mL/min。
微球的载药量 和药物包封率
和药物包封率 计算公式如下:
计算公式如下:
 (1)
(1)
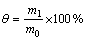 (2)
(2)
式中:m0为实际投药质量;m1为微球中药物质量;m2为称取微球总质量。
1.5 体外释药性能研究
准确称取一定量的载药微球,置于透析袋中(截留相对分子质量为12 000~14 000),再加入5 mL pH 7.4的磷酸盐缓冲液,两端扎紧,加入到45 mL pH=7.4的磷酸盐缓冲液中,并放置于37 ℃恒温振荡浴锅中。微球的释放性能通过测定一定间隔时间的释放介质中的非那雄胺含量来确定。每隔一段时间取出2 mL溶液,同时补充相同体积的缓冲溶液,并用上面描述的HPLC法测定药物的释放量。再以缓冲液中非那雄胺的释放百分比与时间为坐标轴作药物释放曲线。
2 结果与讨论
2.1 制备条件对微球性能的影响
由于非那雄胺为油溶性药物,因此制备PPCM-finasteride微球采用单乳液溶剂挥发法来形成稳定的O/W乳液,并在分离干燥后获得载药微球。在制备过程中油相中PPCM与finasteride的质量比(m(PPCM):m(finasteride))、油相中聚合物含量、乳液搅拌速度、水分散相中稳定剂PVA的含量等因素决定了最终获得的微球的性质。通过实验条件的探索,确定乳液搅拌速度为700 r/min,持续搅拌时间为3 h,油相聚合物PPCM在二氯甲烷(DCM)溶剂中的质量分数为4%,水分散相中稳定剂PVA的质量分数为0.5%。本实验主要考察关键因素PPCM与finasteride质量比对微球性能的影响。实验序号、实验条件及微球特性如表1所示。
表1 不同 m(PPCM):m(finasteride)条件下所制备的微球性能
Table 1 Properties of microspheres prepared at various m(PPCM):m(finasteride)

微球载药量和包封率主要的影响因素是聚合物与药物的质量比,随着非那雄胺含量的增加,微球的载药量提高,而药物的包封率则明显降低。非那雄胺含量的增加使得O/W体系中非那雄胺浓度梯度加大,由于浓度梯度的推动作用,有部分非那雄胺扩散至水相,从而造成药物的损失,而且这一趋势与药物的加入量的增加成正比。也就是随m(PPCM):m(finasteride)的增大,药物在O/W乳液中的浓度减小,因而包封率增大。结果显示,当聚合物与非那雄胺的质量比为5:1时,微球具有较高的载药量与包封率,其载药量在尽可能高的同时,保证了较大的包封率,以减少finasteride的损失。微球的平均粒径随着m(PPCM):m(finasteride)的减小而增大,但变化不明显,这可能与溶于油相的药物浓度减小有关,从而得到粒径更小的微球。
2.2 载药微球的粒度大小与形貌特征
通过光学显微镜观察O/W单相微球,图1(a)所示为PPCM-finasteride微球乳液的光学显微镜照片,所制微球流动性好,微球外观较圆整。表明获得了稳定的、具有良好球形的微球乳液。图1(b)所示为在m(PPCM):m(finasteride)=5:1条件下制备的聚合物微球的扫面电镜图。微球的粒径约为2 μm,微球具有良好的成球性和分散性。微球粒径和粒度分布用激光衍射粒度分析仪测定,所有微球的粒径基本呈单分散正态分布,分布范围较窄。
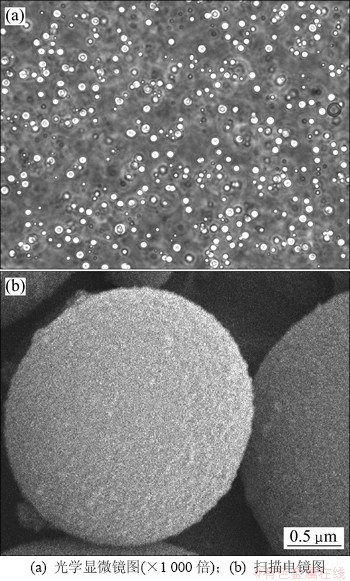
图1 PPCM-finasteride微球的形貌特征图
Fig. 1 Morphological characterization of PPCM-finasteride microsperes
2.3 广角XRD分析
图2(a)所示为finasteride的广角XRD谱,表明finasteride为一种结晶性的药物。图2(b)所示为PPCM-finasteride微球干样的X线衍射谱,显示测试样品为无定型态,没有观察到finasteride相应的衍射峰,说明finasteride充分包裹在无定型聚合物PPCM中[18]。
2.4 微球的体外药物释放性能
PPCM-finasteride微球的体外释药研究在pH为7.4的磷酸盐缓冲溶液中进行。图3(a)所示为微球F2药物释放量与时间的特性关系。药物非那雄胺的释放表现为两阶段模式,即包括突释阶段与突释后的缓慢释放阶段。微球内药物的浓度越高,膜内外药物的浓度差越大,所以表现在药物的初始释放速率较快而且药物释放过程中会产生一些孔道,在初始释药过程中的孔道数较多,内部药物更易通过孔道释放出来,因此表现为前阶段的快速释放和后阶段的缓慢释放。经过42 d的释药后,药物释放量达到(92.59±2.62)%。
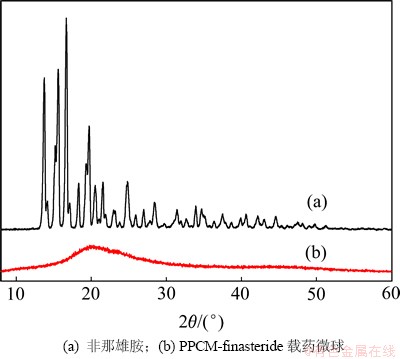
图2 非那雄胺及PPCM-finasteride载药微球的广角XRD谱
Fig. 2 Wide-angle X-ray diffraction spectra of drug finasteride and PPCM-finasteride microspheres
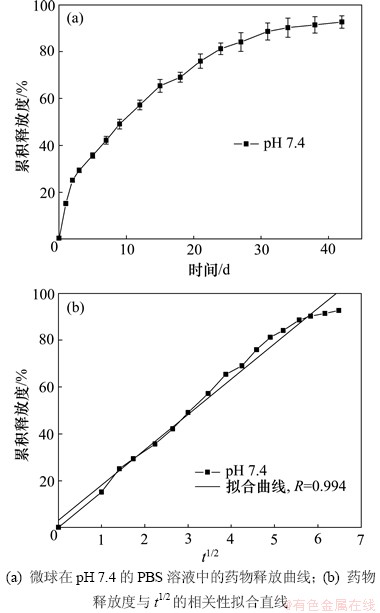
图3 载药微球的释放曲线和拟合直线
Fig. 3 Release profiles of finasteride from drug loaded microspheres and linear fitting chart
图3(b)所示为t1/2与药物finasteride释放量的直线关系,通过用Higuchi方程拟合,直线相关性好。所制微球F2在pH 7.4的PBS溶液中释放规律符合Higuchi方程:Qt=3.11+15.07 t1/2 ,R=0.994。
3 结论
(1) 以非那雄胺为主药,聚合物PPCM为药物载体,采用单乳液(O/W)溶剂挥发法成功地制备了PPCM-finasteride微球。
(2) 探讨了PPCM-finasteride微球制备工艺中各影响因素对PPCM载药性能的影响,通过调节PPCM与finasteride的质量比,可获得较高载药量和包封率的聚合物微球。在优化工艺条件下得到的微球形貌规整,平均粒径约为2 μm。
(3) 微球的体外释药性能研究在pH 7.4的磷酸缓冲溶液中进行,经过42 d的释药,微球F2的累计药物释放量为(92.59±2.62)%,微球的体外释放特性符合Higuchi方程Qt = 3.11+15.07 t1/2 。
(4) 聚合物PPCM可为药物控制释放系统提供了一种新型可降解聚合物,所制备的PPCM-finasteride微球可望作为治疗长效前列腺增生症和男性雄激素性脱发的药物传递系统。
参考文献:
[1] ZHANG Wen, LIN Xiaojie, ZHANG Xingxiang. Biodegradable poly(lactic acid) microspheres containing total alkaloids of caulis sinomenii [J]. Bulletin of Materials Science, 2011, 34 (7): 1715-1719.
[2] Essa S, Rabanel J M, Hildgen P. Characterization of rhodamine loaded PEG-g-PLA nanoparticles (NPs): effect of poly(ethylene glycol) grafting density[J]. International Journal of Pharmaceutics, 2011, 411(1/2): 178-187.
[3] Rafati A, Boussahel A, Shakesheff K M, et al. Chemical and spatial analysis of protein loaded PLGA microspheres for drug delivery applications[J]. Journal of Controlled Release, 2012, 162(2): 321-329.
[4] Makadia H K, Siegel S J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier[J]. Polymers, 2011, 3(3): 1377-1397.
[5] Dhanaraju M D, Gopinath D, Ahmed M R, et al. Characterization of polymeric poly(ε-caprolactone) injectable implant delivery system for the controlled delivery of contraceptive steroids[J]. Journal of Biomedical Materials Research, 2006, 76A(1): 63-72.
[6] 于美丽, 杜智, 王瑞, 等. 聚酸酐-吡柔比星长效植入剂在动物模型体内的抑瘤活性[J].中国组织工程研究, 2012, 16(51): 9595-9599.
YU Meili, DU Zhi, WANG Rui, et al. In vivo antitumor activity of the polyanhydride-pirarubicin long-term implant in animal models[J]. Chinese Journal of Tissue Engineering Research, 2012, 16(51): 9595-9599.
[7] Seow W Y, Yang Y Y. Functional polycarbonates and their self-assemblies as promising non-viral vectors[J]. Journal of Controlled Release, 2009, 139: 40-47.
[8] FENG Jun, ZHUO Renxi, ZHANG Xianzheng. Construction of functional aliphatic polycarbonates for biomedical applications[J]. Progress in Polymer Science, 2012, 37: 211-236.
[9] LIU Suqin, XIAO Han, HUANG Kelong, et al. Terpolymerization of carbon dioxide with propylene oxide and ε-caprolactone: synthesis, characterization and biodegradability[J]. Polymer Bulletin, 2006, 56: 53-62.
[10] Al-Azemi T F, Bisht K S. One-step synthesis of polycarbonates bearing pendant carboxyl groups by lipase-catalyzed ring-opening polymerization[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2002, 40: 1267-1274.
[11] Jeong J H, Kim S W, Park T G. Molecular design of functional polymers for gene therapy[J]. Progress in Polymer Science, 2007, 32: 1239-1274.
[12] Zhang Z, Grijpma D W, Feijen J. Poly(trimethylene carbonate) and monomethoxy poly(ethylene glycol)-block-poly(trimethylene carbonate) nanoparticles for the controlled release of dexamethasone[J]. Journal of Controlled Release, 2006, 111(3): 263-270.
[13] LIU Yanfei, HUANG Kelong, PENG Dongming, et al. Synthesis, characterization and hydrolysis of an aliphatic polycarbonate by terpolymerization of carbon dioxide, propylene oxide and maleic anhydride[J]. Polymer, 2006, 47(26): 8453-8461.
[14] Roehrborn C G. 5-alpha-reductase inhibitors prevent the progression of benign prostatic hyperplasia[J]. Reviews in Urology, 2003, 5: S12-S21.
[15] Coltman C A Jr, Thompson I M Jr, Feigl P. Prostate cancer prevention trial (PCPT) update[J]. European Urology, 1999, 35: 544-547.
[16] Freiberg S, Zhu X X. Polymer microspheres for controlled drug release[J]. International Journal of Pharmaceutics, 2004, 282(1/2): 1-18.
[17] PENG Dongming, HUANG Kelong, LIU Yanfei, et al. Preparation of carbon dioxide/propylene oxide/ε-caprolactone copolymers and their drug release behaviors[J]. Polymer Bulletin, 2007, 59: 117-125.
[18] LIU Yanfei, HUANG Kelong, PENG Dongming, et al. Preparation of poly(butylene-co-ε-caprolactone carbonate) and their use as drug carriers for a controlled delivery[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2007, 45(11): 2152-2160.
(编辑 杨幼平)
收稿日期:2013-10-12;修回日期:2014-01-20
基金项目:湖南省自然科学基金资助项目(13JJ3099);湖南省自然科学-株洲联合基金资助项目(12JJ9046);中南大学中央高校基本科研业务费专项资金资助项目(2013zzts176)
通信作者:刘艳飞(1969-),女,湖南邵阳人,博士,副教授,从事药物载体材料与药物制剂研究;电话:13017381167;E-mail: liuyf@csu.edu.cn
摘要:以新型聚合物聚碳酸亚丙酯马来酸酯(PPCM)为载体,采用Oil/Water单乳液溶剂挥发法制备药物非那雄胺(finasteride)的缓释微球,并研究聚合物PPCM与药物finasteride的质量比对微球特性的影响。研究结果表明:所得PPCM微球外观圆整,平均粒径约为2 μm。随着非那雄胺比例的增加,微球的载药量提高,而药物的包封率则明显降低。在m(PPCM):m(finasteride)为5:1的条件下,获得较高的载药量和包封率,分别为14.78%和66.17%。在pH 7.4的磷酸盐缓冲溶液中,载药微球的体外释放时间达42 d,药物累积释放量为(92.59±2.62)%。微球的释药特性符合Higuchi方程Qt = 3.11+15.07 t1/2。PPCM适用于长效缓释药物传递系统。


