
Structure and antibacterial activity of new layered perovskite compounds
TAN Shao-zao(谭绍早)1, ZHANG Li-ling(张力玲)1, XIA Liao-yuan(夏燎原)1,
LIU Ying-liang(刘应亮)1, LI Du-xin(李笃信)2
1. Department of Chemistry, Ji’nan University, Guangzhou 510632, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 17 December 2006; accepted 2 February 2007
Abstract:
New layered perovskite compounds, AgxNa2-xLa2Ti3O10 (x=0.2, 0.3 and 0.5) were synthesized by an ion-exchange reaction of Na2La2Ti3O10 with AgNO3 solution and characterized by energy dispersive X-ray analysis(EDX), X-ray diffractometry(XRD), scanning electron microscopy(SEM) and X-ray photoelectron spectroscopy(XPS). The ion-exchange processes were optimized, and the antibacterial activity, light permanency and water-resistance were evaluated. Surprisedly, no significant changes in crystal structure of Na2La2Ti3O10 are found by the exchange of silver ions. The Ag0.3Na1.7La2Ti3O10 particles conglomerate obviously with irregular shape and size. Ag0.3Na1.7La2Ti3O10, possessing the minimum inhibitory concentrations(MICs) against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) of 180 mg/L and 240 mg/L, has high antibacterial activity, good light permanency and water-resistance. The ionic state silver in AgxNa2-xLa2Ti3O10 is the antibacterial active component.
Key words:
layered perovskite compound; structure; antibacterial activity; ion-exchange;
1 Introduction
It is widely known that inorganic antibacterial agents carrying silver have high antibacterial activity to microorganisms[1-4] and display no or little side effects on tissue[5]. The applications of the inorganic antibacterial agents are limited because of discoloration and high cost though they are used as sterilizing and antibacterial agents in paper, plastic, paint, ceramic and so on. Therefore, it is important to develop new antibacterial agents with low cost, high antibacterial activity and light permanency.
Layered perovskite compounds with Ruddlesden- Popper phase of ![]() where A is an exchangeable alkali metal cation, A′ is an alkali metal, alkaline earth, main group and/or rare earth, and B is a transition metal, have been investigated extensively in recent years[6-7]. These compounds possessing interesting structure and ion-exchange property have been explored as ionic conductors[8-9], photocatalysts [10-12], and super-conductors[13]. However, there have been few reports on the study of the antibacterial activity, and the influence of silver valence state on antibacterial activity is not clear so far. In this paper, new layered perovskite compounds, AgxNa2-xLa2Ti3O10 (x=0.2, 0.3 and 0.5) carrying low content silver were synthesized and characterized. And the antibacterial activity, light permanency and water-resistance of the compounds were investigated.
where A is an exchangeable alkali metal cation, A′ is an alkali metal, alkaline earth, main group and/or rare earth, and B is a transition metal, have been investigated extensively in recent years[6-7]. These compounds possessing interesting structure and ion-exchange property have been explored as ionic conductors[8-9], photocatalysts [10-12], and super-conductors[13]. However, there have been few reports on the study of the antibacterial activity, and the influence of silver valence state on antibacterial activity is not clear so far. In this paper, new layered perovskite compounds, AgxNa2-xLa2Ti3O10 (x=0.2, 0.3 and 0.5) carrying low content silver were synthesized and characterized. And the antibacterial activity, light permanency and water-resistance of the compounds were investigated.
All reagents used were of analytical purities. The parent compound (carrier) of Na2La2Ti3O10 was prepared by conventional solid-state reaction[9,14]. The raw materials of sodium carbonate, lanthanum oxide, and titanium oxide were mixed at the mole ratio of 1.6?2?3. A 60% (mole fraction) stoichiometric excess of sodium carbonate was added to compensate for the loss due to the evaporation of the sodium component, which can act as an oxidizing flux also[15]. The mixture was ground into powder, and heated at 105 ℃ for 10 h in air. The obtained powder was placed into alumina crucible, calcined in air at 500 ℃ for 10 h, heated at 1 000 ℃ for another 5 h, and then cooled. At last, the product was washed with deionized water and dried at 105 ℃ for 10 h.
AgxNa2-xLa2Ti3O10 was synthesized by an ion- exchange method. 1 mol Na2La2Ti3O10 was added into deionized water to obtain a 10% suspension, and intermixed with 0.1-0.3 mol AgNO3, and then maintained at 40-70 ℃ for 1-7 h in a dark vessel. The products were filtered out, washed with deionized water, and dried at 105 ℃ for 10 h. After grinding and sieving, the portion with average size of 5 μm was used in property tests.
The Ag+ content in reaction solution was measured by a HITACHI 180-80 atomic absorption spectro- photometer. After a certain time of an ion-exchange reaction, the Ag+ exchange fraction (η(Ag+)) was calculated according to the following equation:
η(Ag+)=m1(Ag+)/m2(Ag+)×100% (1)
where m1(Ag+) and m2(Ag+) are the mass fraction of Ag+ in AgxNa2-xLa2Ti3O10 and initial reaction solution, respectively.
The crystal structures of the samples were characterized by XRD on a MASL-XD2 X-ray diffractometer using Cu Kα radiation (36 kV, 24 mA, λ=0.154 nm), and a scanning rate of 0.08 (?)/s was used to record the patterns in the range of 5?-65?; the components and silver content were analyzed by EDX using an Oxford ISIS-300 energy-dispersive X-ray detector; the surface morphologies of Na2La2Ti3O10 and Ag0.3Na1.7La2Ti3O10 were observed using a JSM-6360 scanning electron microscope; the silver valence state in Ag0.3Na1.7La2Ti3O10 was determined with an ESCALab220i-XL electron spectrometer.
The antibacterial activity (minimum inhibitory concentrations, MICs) of Na2La2Ti3O10 and AgxNa2-xLa2- Ti3O10 was estimated by a two-fold diluting method[16], and the bacteria of E. coli ATCC25922 and S. aureus ATCC6538 were selected as indicators. The light permanency was carried out by exposing the samples under fluorescent ultraviolet lamp (351 nm) for a certain time and observing the discoloration. 0.1 g Ag0.3Na1.7La2Ti3O10 was soaked in 20 mL deionized water in a polypropylene bottle at 37 ℃ and rotated with a diameter of 3 cm and a rotating speed of 120 r/min. After 1-10 d of rotation, the Ag+ concentration released in water was measured by a HITACHI 180-80 atomic absorption spectrophotometer.
3 Results and discussion
3.1 Ion-exchange process
The antibacterial activity of AgxNa2-xLa2Ti3O10, which is directly associated with the silver content, is affected by the ion-exchange process. The influences of reaction temperature, reaction time, AgNO3 dosage on Ag+ exchange fraction (or the silver content of AgxNa2-xLa2Ti3O10) were investigated as follows.
When the mole ratio of Ag to Na in initial reaction solution was 0.2?1, Ag+ exchange fraction was determined as functions of reaction temperature (40-70℃) and reaction time (up to 7 h). As shown in Fig.1, Ag+ exchange fraction increases with the increase of reaction temperature and reaction time. Furthermore, at relatively high temperature (60-70 ℃), Ag+ exchange fraction levels off above 6 h. The results suggest that the ion- exchange reaction reaches a high Ag+ exchange fraction at 60 ℃ for 6 h.

Fig.1 Effect of reaction temperature and reaction time on Ag+ exchange fraction (η(Ag+))
Ag+ exchange fraction and the mass fraction of Ag+ in AgxNa2-xLa2Ti3O10 as functions of the mole ratio Ag to Na in initial reaction solution at 60 ℃ for 6 h are shown in Fig.2. With the increase of mole ratio of Ag to Na, Ag+ exchange fraction declines and the mass fraction of silver increases. Surprisedly, a further increase in mole ratio of Ag to Na (beyond 0.2) led to a sharp decline in Ag+ exchange fraction. It may be explained that the silver ion concentration on carrier surface tended to rise with the increase of mole ratio of Ag to Na, and then more silver ions exchanged with carrier causing the increase of mass fraction of Ag+. At the same time, the silver ions remaining in solution might be reduced to metallic silver, which would choke exchange sites when they deposited on carrier surface. Therefore, with the increase of mole ratio of Ag to Na, more metallic silver particles and more choked exchange sites result in the decrease of Ag+ exchange fraction.

Fig.2 Effect of mole ratio of Ag to Na on Ag+ exchange fraction and mass fraction of Ag+ in AgxNa2-xLa2Ti3O10
According to the aforementioned results, the optimized ion exchange process is as follows: reaction temperature 60 ℃, reaction time 6 h, mole ratio of Ag to Na 0.2.
3.3 Structure analysis
The results of EDX analysis for Na2La2Ti3O10 and AgxNa1-xLa2Ti3O10 are shown in Table 1. According to the mole ratio of Na, La and Ti, the molecular formula of Na2La2Ti3O10 (sample 1) can be confirmed. The molecular formulae of AgxNa1-xLa2Ti3O10 (samples 2, 3 and 4) can also be determined because the mole ratios of Ag, Na, La and Ti are almost in accordance with the molecular formulae of Ag0.2Na1.8La2Ti3O10, Ag0.3Na1.7- La2Ti3O10 and Ag0.5Na1.5La2Ti3O10.
Table 1 Results of EDX analysis for Na2La2Ti3O10 and AgxNa1-xLa2Ti3O10 (mole fraction, %)

The XRD patterns of Na2La2Ti3O10 and AgxNa1-xLa2Ti3O10 (x= 0.2, 0.3 and 0.5) are shown in Fig.3. Obviously, the diffraction peaks of AgxNa2-xLa2Ti3O10 (x=0.2, 0.3 and 0.5) are almost the same as that of Na2La2Ti3O10, which are consistent with the earlier determined pattern of compound Na2La2Ti3O10 with tetragonal crystalline[17]. However, small changes are observed in the unit cell parameters of AgxNa1-xLa2Ti3O10 (x= 0.2, 0.3 and 0.5) because of the different sizes between Na+ (0.098 nm) and Ag+ (0.113 nm), as can be seen in Table 2. As a result, no significant changes in crystal structure of Na2La2Ti3O10 are found by the exchange of silver ions.

Fig.3 XRD patterns of Na2La2Ti3O10 and AgxNa1-xLa2Ti3O10
Table 2 Unit cell parameters of Na2La2Ti3O10 and AgxNa2-x- La2Ti3O10
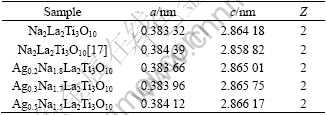
The SEM images of Na2La2Ti3O10 and Ag0.3Na1.7La2Ti3O10 are shown in Fig.4. Fig.4(a) corresponding to Na2La2Ti3O10 particles display some conglomeration with flocculent shape and uneven size due to the high temperature solid-state reaction. Moreover, Ag0.3Na1.7La2Ti3O10 particles conglomerate obviously with irregular shape and size because of the import of silver ions, as shown in Fig.4(b).
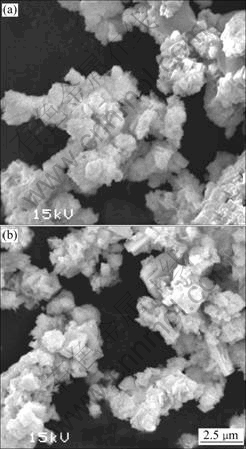
Fig.4 SEM images of Na2La2Ti3O10 (a) and Ag0.3Na1.7La2Ti3O10 (b)
3.3 Properties evaluation
The antibacterial activity and light permanency of Na2La2Ti3O10 and AgxNa2-xLa2Ti3O10 (x=0.2, 0.3 and 0.5) are listed in Table 3. The MICs of AgxNa2-xLa2Ti3O10 against E. coli and S. aureus are all less than 300 mg/L, in contrast with that of higher than 2 g/L for Na2La2- Ti3O10. On the other hand, Na2La2Ti3O10, Ag0.2Na1.8La2- Ti3O10 and Ag0.3Na1.7La2Ti3O10 show no discoloration while Ag0.5Na1.5La2Ti3O10 displays a little discoloration after exposing to UV light for 24 h. These results indicate that the antibacterial activity of AgxNa2-xLa2- Ti3O10 improves with the increase of silver content or the value of x. But at the same time, the trend to discoloration increases. It is interesting that the MICs of Ag0.3Na1.7La2Ti3O10 against E. coli and S. aureus are 180 mg/L and 240 mg/L, respectively, and its discoloration is not observed after exposing to UV light for 24 h. Therefore, Ag0.3Na1.7La2Ti3O10 has the potential to be commercially used in future.
Table 3 Antibacterial activity and light permanency of Na2La2Ti3O10 and AgxNa2-xLa2Ti3O10 (MIC/(mg?L-1))

Fig.5 shows the mass fraction of release silver ions for Ag0.3Na1.7La2Ti3O10 as a function of soaking time at 37 ℃ in deionized water. In the first two days, a large amount of silver ions rapidly releasing into water were detected. With increasing soaking time, the rate of release silver ions slows down. Surprisedly, the mass fraction of release silver ions is only 6.5% (mass fraction) after soaking for 10 d. The results indicate that Ag0.3Na1.7La2Ti3O10 shows good water-resistance.

Fig.5 Relation between mass fraction of release silver ions for Ag0.3Na1.7La2Ti3O10 in deionized water and soaking time
3.4 Exploration of antibacterial active component
The silver valence state in Ag0.3Na1.7La2Ti3O10 was analyzed by XPS, and the Ag 3d spectrum is shown in Fig.6. The binding energies of Ag 3d5/2 and Ag 3d3/2 are 367.59 eV and 373.63 eV, respectively, which are in
accordance with standard parameters of ionic silver[18]. This result suggests that the silver in Ag0.3Na1.7La2Ti3O10 exists as ionic state.
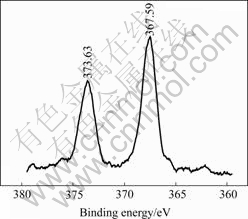
Fig.6 Ag 3d spectrum of Ag0.3Na1.7La2Ti3O10
The effect of silve valence state on antibacterial activity was further estimated as shown in Table 4. With the lapse of UV irradiation time, for Ag0.3Na1.7La2Ti3O10, the MICs increase and the discoloration becomes serious. These results can be explained that when Ag0.3Na1.7La2Ti3O10 is exposed to UV light, some ionic silver is reduced to metallic silver, and the metallic silver content in Ag0.3Na1.7La2Ti3O10 increases with the increase of UV irradiation time. Unfortunately, the color becomes deep, and the antibacterial activity declines.
Table 4 MICs and discoloration of Ag0.3Na1.7La2Ti3O10 at different UV irradiation time (MIC/(mg?L-1))

Therefore, it is evident that the ionic state silver in AgxNa2-xLa2Ti3O10 is the antibacterial active component.
4 Conclusions
1) New layered perovskite compounds, AgxNa2-xLa2Ti3O10 (x=0.2, 0.3 and 0.5) are synthesized by an ion-exchange reaction of Na2La2Ti3O10 with AgNO3 solution; the optimized ion exchange process is as follows: reaction temperature 60 ℃, reaction time 6 h, mole ratio of Ag to Na 0.2.
2) The molecular formulae of AgxNa2-xLa2Ti3O10 (x=0.2, 0.3 and 0.5) are confirmed; no significant changes in crystal structure of Na2La2Ti3O10 are found by the exchange of silver ions; Ag0.3Na1.7La2Ti3O10 particles conglomerate obviously with irregular shape and size.
3) Ag0.3Na1.7La2Ti3O10, possessing the MICs against E. coli, S. aureus of 180 mg/L and 240 mg/L, has high antibacterial activity, good light permanency and water-resistance.
4) The ionic state silver in AgxNa2-xLa2Ti3O10 is the antibacterial active component.
References
[1] AYBEN T, SEMRA ?. Silver, zinc, and copper exchange in a Na-clinoptilolite and resulting effect on antibacterial activity [J]. Applied Clay Science, 2004, 27(1/2): 13-19.
[2] GARZA M R, OLGU?N M T, SOSA I G, ALC?NTARA D, FUENTES G R. Silver supported on natural mexican zeolite as an antibacterial material [J]. Microporous and Mesoporous Materials, 2000, 39: 431-444.
[3] ZHANG S, FU R, WU D, XU W, YE Q, CHEN Z. Preparation and characterization of antibacterial silver-dispersed activated carbon aerogels [J]. Carbon, 2004, 42: 3209-3216.
[4] JEON H J, YI S C, OH S G. Preparation and antibacterial effects of Ag-SiO2 thin films by sol-gel method [J]. Biomaterials, 2003, 24: 4921-4928.
[5] KAWASHITA M, TSUNEYAMA S, MIYAJI F, KOKUBO T, KOZUKA H, YAMAMOTO K. Antibacterial silver-containing silica glass prepared by sol-gel method [J]. Biomaterials, 2000, 21: 393-398.
[6] GOPALAKRISHNAN J, SIVAKUMAR T, RAMESHA K, THANGADURAI V, SUBBANNA G N. Transformations of Ruddlesden-Popper oxides to new layered perovskite oxides by metathesis reactions [J]. Journal of American Chemical Society, 2000, 122(26): 6237-6241.
[7] PATRAKEEV M V, LEONIDOV I A, KOZHEVNIKOV V L, KHARTON V V. Ion-electron transport in strontium ferrites: Relationships with structural features and stability [J]. Solid State Sciences, 2004, 6(9): 907-913.
[8] TODA K, WATANABE J, SATO M. Crystal structure determination of ion-exchangeable layered perovskite compounds, K2La2Ti3O10 and Na2La2Ti3O10 [J]. Mater Res Bull, 1996, 31(11): 1427-1435.
[9] TODA K, WATANABE J, SATO M. Synthesis and ionic conductivity of new layered perovskite compounds, Ag2La2Ti3O10 [J]. Solid State Ionics, 1996, 90(1/4): 15-19.
[10] DOMEN K, YOSHIMURA J, SEKINE T, TANAKA A, ONISHI T. A novel series of photocatalysts with an ion-exchangeable layered structure of niobate [J]. Catal Lett, 1990, 4(4/6): 339-343.
[11] TAKATA T, FURUMI Y, SHINOHARA K, TANAKA A, HARA M, KONDO J, DOMEN K. Photocatalytic decomposition of water on spontaneously hydrated layered perovskites [J]. Chem Mater, 1997, 9(5): 1063-1064.
[12] IKEDA S, HARA M, KONDO J, DOMEN K, TAKAHASHI H, OKUBO T, KAKIHANA M. Preparation of K2La2Ti3O10 by polymerized complex method and photocatalytic decomposition of water [J]. Chem Mater, 1998, 10(1): 72-77.
[13] BEDNORZ J G, MULLER K A Z, TAKASHIGE M. Superconductivity in alkaline earth-substituted La2CuO4-y [J]. Science, 1987, 236(4797): 73-75.
[14] BYEON S H, NAM H J. Neutrion diffraction and FT-Raman study of ion-exchangeable layered titanates and niobates [J]. Chem Mater, 2000, 12(6): 1771-1778.
[15] CAVA R J, KRAJEWSKIM J J, PECK JR W F, BATLOGG B, RUPP JR L W, FLEMING R M, JAMES A C W P, MARSH P. Synthesis of bulk superconducting yttrium-barium-copper-oxide (YBa2Cu4O8) at one atmosphere oxygen pressure [J]. Nature, 1989, 388(6213): 328-330.
[16] TAN S Z, ZHANG K H, LIU Y L. Preparation and property of montmorillonite composite materials exchanged with Cu2+ and quaternary phosphonium cation [J]. Acta Materiae Compositae Sinica, 2006, 23(3): 82-86. (in Chinese)
[17] GOPALAKRISHNAN J, BHAT V. A2Ln2Ti3O10 (A=K or Rb; Ln=La or rare earth): A new series of layered perovskites exhibition ion exchange [J]. J Inorg Chem, 1987, 26: 4329-4331.
[18] MOULDER J F, STICKLE W F, SOBOL P E, BOMBEN K D. Handbook of X-Ray Photoelectron Spectroscopy [M]. Eden Prairie Minnesota: Perkin-Elmer, 1992: 120-121.
Foundation item: Projects(20676049, 50472077) supported by the National Natural Science Foundation of China; Projects(05200555; 2004B20201026) supported by the Natural Science Foundation of Guangdong Province of China; Project(2005Z3-D212) supported by the Science and Technology Project Fund of Guangzhou City of China
Corresponding author: TAN Shao-zao; Tel: +86-20-85221813; Fax: +86-20-87692365; E-mail: tanshaozao@163.com
Abstract: New layered perovskite compounds, AgxNa2-xLa2Ti3O10 (x=0.2, 0.3 and 0.5) were synthesized by an ion-exchange reaction of Na2La2Ti3O10 with AgNO3 solution and characterized by energy dispersive X-ray analysis(EDX), X-ray diffractometry(XRD), scanning electron microscopy(SEM) and X-ray photoelectron spectroscopy(XPS). The ion-exchange processes were optimized, and the antibacterial activity, light permanency and water-resistance were evaluated. Surprisedly, no significant changes in crystal structure of Na2La2Ti3O10 are found by the exchange of silver ions. The Ag0.3Na1.7La2Ti3O10 particles conglomerate obviously with irregular shape and size. Ag0.3Na1.7La2Ti3O10, possessing the minimum inhibitory concentrations(MICs) against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) of 180 mg/L and 240 mg/L, has high antibacterial activity, good light permanency and water-resistance. The ionic state silver in AgxNa2-xLa2Ti3O10 is the antibacterial active component.


