
Corrosion characteristics of steel in seawater containing various chloride concentrations generated by electrochemical method
Seong-Jong KIM, Seok-Ki JANG
Division of Marine Engineering, Mokpo Maritime University, Mokpo City, Jeonnam 530-729, Korea
Received 2 March 2009; accepted 30 May 2009
Abstract:
The electro-clean system (ECS), a ballast water management system that eliminates harmful aquatic organisms in ship ballast water via electrochemically produced chloride, was developed. The current density for potentiostatic experiments subjected to seawater with an applied potential (-0.1 V) at various TRC concentrations (2×10-6, 6×10-6, 10×10-6, and 15×10-6) have similar results. The current density at -0.1 V has the highest value compared with that at -0.7 V and -0.4 V. For all concentrations, the current density increases with increasing applied potential.
Key words:
seawater, electro clean system (ECS), TRC concentration, current density;
1 Introduction
When ships discharge the ballast seawater in a different location, they change the local seawater salinity and introduce nonindigenous oceanic organisms and pathogens. The amount of ballast water that ships transport and discharge is estimated to be over 15 billion tons per year, and it is expected to increase as shipping traffic increases. Hence, solving this problem will be a very important issue in the near future. To prevent, minimize, and ultimately eliminate risks to the environment and resources that arise from the transplant of harmful aquatic organisms and pathogens, the International Maritime Organization (IMO) has proposed to control and manage ship ballast water and sediment by regulating ballast water management[1-2]. In United States and Australia, regulations for ship ballast water have been enforced since September 2004. Various solutions, such as electrolysis, ozonation[3], sterilization, disinfection, filt-ration, using a water jet system, etc., are being developed[4-7]. Concern over this issue has become a top priority in the world[8-9].
In this study, the effects of various chloride concentrations on the material used for ship ballast water tanks were evaluated by electrochemical experiments.
2 Experimental
An electrochemical experiment was conducted on samples of carbon steel. A polarization experiment was conducted with a silver/silver chloride electrode (SSCE) as the standard electrode and a platinum electrode as the counter electrode at a scan rate of 2 mV/s under natural seawater conditions. Various chloride concentrations such as 2×10-6, 6×10-6, 10×10-6, and 15×10-6, produced by the ECS, were used. Cathodic polarization and anodic polarization tests were conducted at an open-circuit potential of -2.0 V and 3.0 V, respectively. For each condition, potentiostatic experiments were conducted to evaluate the current density variations during and after 1 200 s. The Tafel analysis was used to calculate the corrosion current density at a polarization value from +0.25 V to -0.25 V, which was based on the open circuit potential. The experiments were conducted more than three times, and their average values were compared with each other.
3 Results and discussion
Fig.1 shows a plot of the natural potential for natural seawater and for seawater containing various total residual chloride (TRC) concentrations produced from the ECS. The noble potential was observed for all immersion conditions. Initially, when immersed in natural seawater with a TRC of 2×10-6, the sample’s potential moves slowly toward a negative value; then, after immersion for approximately 8 ks, the potential value becomes increasingly negative for concentrations between 2×10-6 and 15×10-6. The seawater setup has the greatest noble potential value, but becomes increasingly negative in value with increasing TRC concentration.
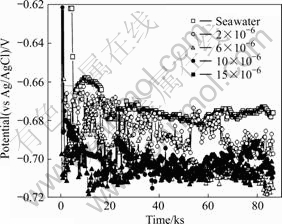
Fig.1 Potential with TRC concentration produced from ECS in seawater
Fig.2 shows the anodic polarization behavior for carbon steels with changing TRC concentration. The potential in seawater has its highest observed value at the open circuit potential. The 2×10-6 and 10×10-6 specimens show similar results in seawater. However, when the potential is greater than 0.1 V, the current densities for all conditions have similar values. From the anodic polarization curve, as the potential increases, the current density also generally increases. Stainless steel and aluminum alloys are usually resistant to corrosion in seawater due to the formation of a passive layer, which is evident in their anodic polarization curves. In this experiment, however, the alloys used do not show passivity characteristics. Due to the presence of the TRC, the anodic polarization behavior is not predictable. Because these samples do not passivate in a seawater environment, any electrochemically defective area on the specimen surface will experience higher current flow compared with the rest of the surface; thus, an increase in the potential produces an increase in the current density [10-13].
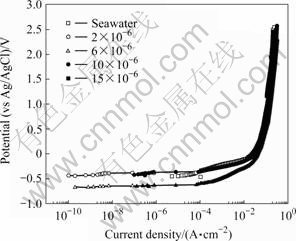
Fig.2 Anodic polarization tendency with TRC concentration
Fig.3 shows the cathodic polarization curves for various TRC concentrations produced from the ECS in natural seawater. The polarization trend is affected by the concentration polarization due to oxygen reduction (O2+2H2O+4e-→4OH-) and the activation polarization due to hydrogen generation (2H2O+2e→H2+2OH-) [14-16]. After the occurrence of concentration polarization, which resulted from the dissolved oxygen reduction reaction below the open circuit potential, the current density rapidly increases at approximately -0.82 V. This behavior is observed for all TRC concentrations except 15×10-6. In seawater, the value is similar to that for 10×10-6 and is very low in 2×10-6 and 6×10-6. The sample current density at 15×10-6 is higher than that for seawater.
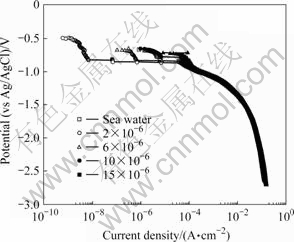
Fig.3 Cathodic polarization tendency with TRC concentration produced by ECS in seawater
Fig.4 plots the current density with time for applied potentials of -0.1, -0.4, and -0.7 V in seawater. It was carried out three times for all conditions. At an applied potential of -0.1 V, the current density starts with a high value, increases for 50 s, and then stabilizes; these results have good reproducibility. At -0.4 V, generally low current densities are observed from the start due to oxidation and reduction reactions occurring alternatively. Thus, the current density after 1 200 s has various values. At -0.7 V, on the other hand, the current density is approximately 4×10-4 A/cm2 initially, and tends to stabilize and decrease with time.
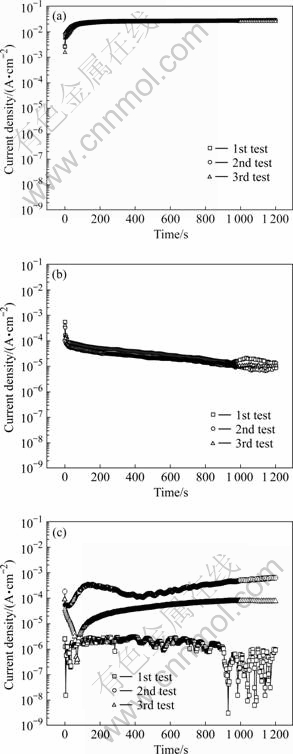
Fig.4 Current density with time when potentiostatic experiment conducted at -0.1 (a), -0.4 (b), and -0.7 V (c) in seawater
Fig.5 compares the average current density values at 1 200 s and applied potentials of -0.1, -0.4, and -0.7 V for tests conducted in seawater at various TRC concentrations. The highest current density at -0.7 V is observed for a concentration of 2×10-6. The lowest current density occurs for a concentration of 15×10-6. However, because the current density repeatedly rose and fell, conclusions can not be reasonably formed for just the current density. The current density for an applied potential of -0.4 V shows significant differences at different TRC concentrations. At -0.1 V, a test performed at a concentration of 2×10-6 is expected to have the least amount of corrosion because it has the lowest current density; the next lowest appears to be seawater. These results are opposite to those at -0.7 V. On the other hand, similar results are obtained for nearly every concentration at -0.1 V, and the highest value is observed at 15×10-6. Except at -0.7 V, the highest current density occurs for tests at a concentration of 15×10-6, which has the highest chloride concentration level. It can be inferred that corrosion will increase with a higher current density; however, this does not adversely affect the ECS because micro-organisms can be eliminated at concentrations below 10×10-6. For each concentration condition, the current density steadily increases with increasing the applied potential. This behavior is similar to the anodic polarization behavior where the current density increases with an increase in potential.
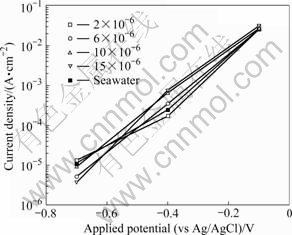
Fig.5 Average value of current densities after potentiostatic experiment for 1 200 s
Fig.6 shows the surface morphologies of sample after a potentiostatic experiment at various TRC concentrations. Generally, a noticeably high corrosive activity occurs at -0.1 V compared with the corrosive activities for other conditions. In seawater, corrosion with areas of partial corrosion occurs over the entire surface. Partial corrosion is observed for the 2×10-6 condition. At 6×10-6, the dissolution reaction corrodes the upper portion while corrosion products are observed in the lower portion. For the 10×10-6 condition, both noticeable corrosion and little dissolution reaction are observed. When comparing the other conditions with the 15×10-6 case, general corrosion is noticeable. At -0.4 V, a slight dissolution reaction is observed in seawater; the least amount of corrosion occurs at 2×10-6; and a significant amount of corrosion occurs at TRC concentrations over 6×10-6. But, the corrosion at 2×10-6 is less than that in seawater. At -0.7 V, corrosion products are observed in the seawater, corrosion by dissolution reaction is barely observed at 2×10-6 and 6×10-6; and a slight amount of corrosion, such as pitting and its products, are observed at 10×10-6 and 15×10-6. In general, as the applied potential increases, the amount of corrosion increases, thus validating the research results from the potentiostatic experiments at various applied potentials.
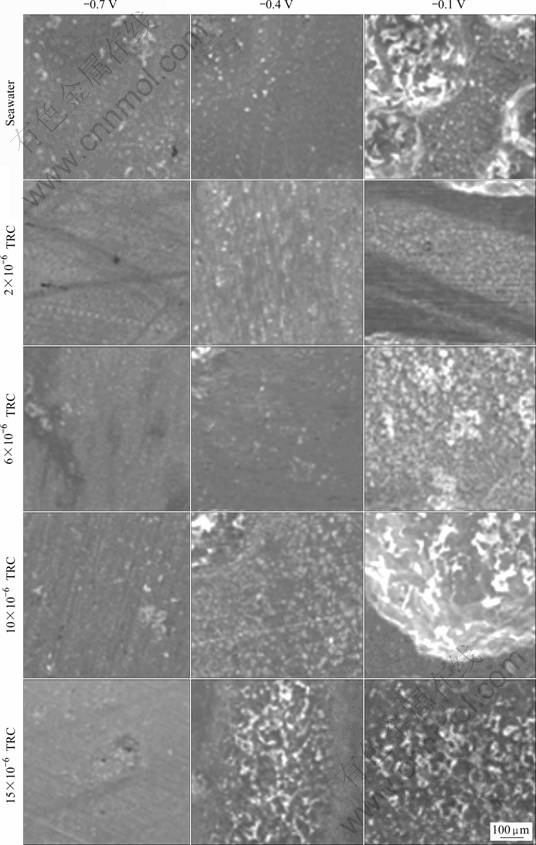
Fig.6 Surface morphologies after potentiostatic experiment with various concentrations of TRC
Fig.7 depicts the polarization curves at 15×10-6 and the corrosion current density obtained by the Tafel analysis. For various TRC values, the analysis was performed at least three times, and then the average corrosion current density was determined. The corrosion current density in seawater increases as the initial delay time increases since the electrochemical reaction takes longer to occur. The increase in corrosion current density indicates a high corrosion rate. The corrosion current density at 2×10-6 is lower than that for seawater conditions, while the corrosion current density at 6×10-6 and 10×10-6 is higher than that for seawater. Thus, TRC concentrations less than 10×10-6 should have similar corrosion current density values to those in seawater conditions. The 15×10-6 density, however, is slightly higher than other values. Because conditions are usually kept at a TRC of less than 10×10-6, the damage from a greater TRC value is nonexistent.
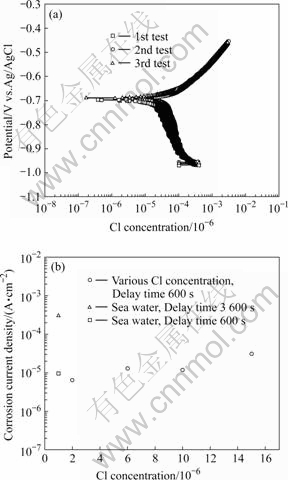
Fig.7 Polarization curves in 15×10-6 (a) and corrosion current density (b) obtained by Tafel analysis
4 Conclusions
From potential measurements, seawater has the highest noble potential. But, as the TRC concentration increases, the potential becomes negative. Except at 15×10-6, the cathodic polarization behavior consists of the dissolved oxygen reduction reaction producing concentration polarization; then, the potential tends to rapidly increase to approximately -0.82 V. The current density at -0.1 V has the highest value compared with that at -0.7 V and -0.4 V. For all concentrations, the current density increases for increasing applied potential. Similar behaviors are observed for the anodic polarization curve. The corrosion behavior at TRC concentrations less than 10×10-6 has similar values to those in seawater.
References
[1] KIM S J, JANG S K, LEE S J, HAN M S, PARK Y S. Evaluation of characteristics for Zn primer coating with chloride concentration [C]// Proceedings of the Korean Society of Marine Engineering, 2008 Spring Conference, 2008: 313-315.
[2] KIM S J, LEE S J, JANG S K. Evaluation of electrochemical characteristics in sea water for KR-RA steel [C]// Proceedings of the Korean Society of Marine Engineering 2008 Autumn Conference, 2008: 243-244.
[3] DNV. Ballast water treatment by ozonation [R]. DNV, 2000: 2000-3229.
[4] DRAGSUND E, JIHANNESSEN B O, ANDERSEN A B, NOKLEBYE J O. Corrosion effects of ballast water treatment methods [C]// 2nd International ballast water treatment R&D Symposium, 2000: 291-299.
[5] MATSUDA M, KOBAYASHI S, MIYUKI H, YOSHIDA S. An anticorrosion method for ballast tanks using nitrogen gas [R]. Ship and Ocean Foundation Technical Report, 1999.
[6] National Research Council. Stemming the tide: Controlling introductions of nonindigenous species by ship’s ballast water [M]. Washington D. C: National Academy Press, 1996.
[7] TAMBURRI M N, WASSON K, MATSUDA M. Ballast water deoxygenation can prevent aquatic introductions while reducing ship corrosion [J]. Biological Conservation, 2002, 103: 331-341.
[8] KIM S J, HAN M S, BAIK S Y, LEE K S, PARK Y S. Electrochemical characteristics of steel with chloride concentration by electro clean system in sea water [C]// Proceedings of the Korean Society of Marine Engineering 2008 Spring Conference, 2008: 363-365.
[9] KIM S J, LEE S J, BAIK S Y. Evaluation of electrochemical characteristics in sea water in Zn primer coated for rudder [C]// Proceedings of the Korean Society of Marine Engineering, 2008: 245-246.
[10] KIM S J, OKIDO M, MOON K M. An electrochemical study of cathodic protection of steel used for marine structures [J]. The Korean Journal of Chemical Engineering, 2003, 20(3): 560-565.
[11] KIM S J, OKIDO M, MOON K M. The electrochemical study on mechanical and hydrogen embrittlement properties of HAZ part as a function of post-weld heat treatment in SMAW [J]. Surface and Coatings Technology, 2003, 169/170: 163-167.
[12] MOON K M, LEE M H, KIM K J, KIM S J. The effect of post-weld heat treatment affecting corrosion resistance and hydrogen embrittlement of HAZ part in FCAW [J]. Surface and Coatings Technology, 2003, 169/170: 675-678.
[13] MOON K M, LEE M H, KIM K J, KIM J G, KIM S J. A study on the post-weld heat treatment effect to mechanical properties and hydrogen embrittlement for heat affected zone of a RE 36 steel [J]. Corrosion Science and Technology, 2003, 2(6): 283-288.
[14] KIM S J, JANG S K, KIM J I. Effects of post-weld heat treatment on optimum cathodic protection potential of high-strength steel in marine environment conditions [J]. Materials Science Forum, 2005, 486/487: 133-136.
[15] KIM S J, JANG S K, KIM J I. Characteristics evaluation of thin films formed in Mg-Al alloy in various chemical conversion solution conditions [J]. Journal of the Korean Society of Marine Engineers, 2005, 29(1): 98-106.
[16] KIM S J, KIM J I. Effects of anodizing time on anodizing of Mg-Al alloy in alkaline solution [J]. Materials Science Forum, 2006, 510/511: 162-165.
Corresponding author: Seong-Jong KIM; Tel: +82-61-2407226; Fax: +82-61-2407201; E-mail: ksj@mmu.ac.kr
(Edited by ZHAO Jun)


