
Preparation of Ti-Al alloy sheet by
electron beam physical vapor deposition
MA Li(马李), HE Xiao-dong(赫晓东), SUN Yue(孙跃)
Center for Composite Material, Harbin Institute of Technology, Harbin 150001, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Ti-Al thin sheet with dimension of 450 mm×450 mm×0.2 mm was prepared by electron beam physical vapor deposition(EB-PVD) technology. The surface and cross-section pattern of as-deposited sample were studied by SEM and AFM, and then the composition and phase were analysed by XRD and EPMA. Finally, the effect on deposit by re-evaporation of Al was explored by calculating the ratio of re-evaporating capacity with depositing capacity of Al on the substrate. The results indicate that the evaporation process with Nb addition into the molten pool makes it earlier to reach the steady-state. The existing equiaxed crystal and columnar crystal along the cross-sectional may be caused by the transformation latent heat released during the transition course of atoms from gaseous state to solid state. The effect on deposit by re-evaporation of Al can be neglected because the re-evaporating capacity of Al is far below that of the depositing capacity.
Key words:
electron beam physical vapor deposition; Ti-Al thin sheet; microstructure; saturation vapor pressure; re-evaporation;
1 Introduction
Ti-Al intermetallic compounds have received considerable attention due to their low density, high specific modulus, and relatively good strength retention at elevated temperatures[1-3]. These properties make Ti-Al intermetallic a potential attractive material for high temperature space and aerospace applications such as thermal protective skin materials for reusable hypersonic vehicles[4-6].
Due to the intrinsic brittleness, it is difficult to prepare Ti-Al thin sheet with thickness below 0.3 mm by the traditional process methods such as rolling process and powder metallurgy technique[7]. Magnetron sputtering technique can be used to synthesize materials with nanometer dimension, but the relative lower deposition rate restricts its application to prepare materials with large size[8]. In this work, electron beam-physical vapor deposition(EB-PVD) technology has been introduced to prepare Ti-Al alloy thin sheet with dimension of 450 mm×450 mm×0.2 mm successfully. EB-PVD makes possible both the deposition of materials that have non-equilibrium chemistries and microstructures, and the manufacture of components having geometries that can not be achieved by more conventional techniques.
EB-PVD technology is currently widely used in the preparation of high-temperature structural materials and structural component. For example, GUAN and HE[9] have prepared Ni-20Cr-0.6Al alloy sheet about 0.2 mm thickness and its room temperature strength reaches 1 685 MPa. Moreover, the Fe-19Cr-5Al-0.2Ti-0.8Y2O3 (PM2000) alloy sheet prepared by EB-PVD can be used as s surface board of thermal protective system and its maximum static oxidation resistance temperature is 1 315 ℃[10]. The overseas research on EB-PVD is mainly focused on the preparation of micro-laminate materials used as hot-end component with complex shapes such as turbine blades. GE Company and the Oak Ridge National Laboratory have been engaged in the study of preparing Nb-Si and Mo-Si micro-laminate with EB-PVD technique[11].
In order to obtain lightmass and high strength structural material used at 500 ℃ to 800 ℃, Ti-Al alloy was selected as subject due to its excellent properties and potential application in the fields of aerospace. The present work describes the process of preparing Ti-Al thin sheet by high-power EB-PVD, and then the micro structure and the phase were studied by SEM, AFM and XRD. Finally, the effect on deposition by re-evaporation of aluminum from substrate was analyzed.
2 Experimental
The primary source material used in this study was Ti-Al alloy in cylindrical shape of about d98.5 mm×300 mm. The alloy has a nominal composition of Ti-48Al in mole fraction.
The experimental equipment used in this study was L5 type EB-PVD apparatus with horizontal feed mode. A schematic of the physical set-up as shown in Fig.1 illustrates the process of evaporation and deposition. One of the crucibles contains the Ti-Al alloy evaporation source. A stainless steel substrate with a certain vertical distance from the crucible may be preheated by the EB-gun above the substrate and the temperature regulation can be carried out by changing the current flow. During the preparation process, the temperature and the rotational speed of the substrate were fixed at 1 000 K and 8 rad/min. In order to increase the heat efficiency during the process of evaporation, a heat protective shield was mounted on the appropriate position in the vacuum chamber. The description of principle for EB-PVD method and equipment can be referred to correlative literatures[12-13].
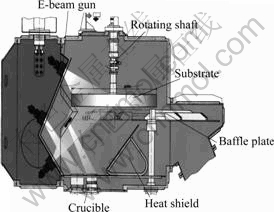
Fig.1 Schematic of EB-PVD apparatus
Calcium fluoride separating layer was deposited onto the substrate before process to make it easier to release the deposit from substrate. Finally, the prepared material with dimension of 450 mm×450 mm×0.2 mm was gained by mechanical stripping from the substrate surface when cooled to 423 K.
X-ray diffraction(XRD) was used to analyze the phase composition of sample after diffusion annealing treatment. Scanning electron microscopy(SEM) and atom force microscopy(AFM) were used to investigate the morphology characteristics of surface and cross-section of the products.
3 Results and discussion
3.1 Microstructures
The surface topography of prepared sample is shown in Fig.2. It can be seen that the grains with a low size-uniformity ranging from 200 nm to 500 nm distribute on the surface randomly, and there are a small number of micropores with macro gaps along the grain boundaries on the surface. AFM image of surface shows that the alloy may grow up with the island mode during the process of sedimentation. There are many slender columnar grains distributed in each island and the average size of the grains is about 300 nm.
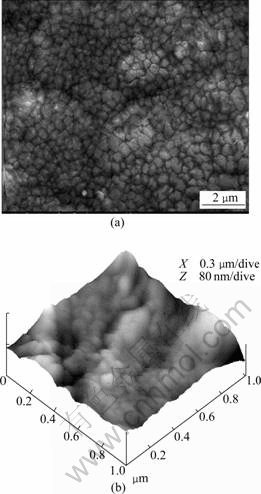
Fig.2 Surface micrographs of as-deposited sample: (a) SEM image; (b) AFM image
The main cause to lead to such a small grain size is due to the high cooling velocity of EB-PVD technology. Gas phase particles will change into solid phase particles directly without the liquid phase transition, and there contains the processes of deposition or absorption, surface diffusion and volume diffusion of gas phase atoms. As the temperature of substrate is so low compared with the evaporation temperature, the diffusion process is so insufficient that it is difficult for grains to grow up on the basis of homogeneous nucleation by the mode of volume diffusion of atoms[14]. Moreover, the evaporation atoms will nucleate primarily at the blemish position on the substrate surface with a high degree of roughness and, the subsequent atoms with higher deposition rates which have no time to diffuse onto the positions with lower potential barrier will nucleate without the process of choosing optimum orientation.
The cross-sectional morphology of as-deposited material is shown in Fig.3, in which there exist two distinctive layers composed of different grains along the thickness direction. Zone ? which is close to the substrate contains columnar crystal with obvious straight boundary among the grains, and zone Ⅱ which is far from the substrate is composed of equiaxed grains. Theoretically, when the temperature of the substrate is 700 ℃ during the deposition process, it should obtain columnar grain according to the established model by MOVCHEN and DEMCHISHIN[15]. The abnormal microstructure may be caused by the transformation latent heat released during the transition course of atoms from gaseous state to solid state, and this transition course will increase the temperature of the substrate, and the equiaxed grain will form when the substrate temperature reaches adequate value.
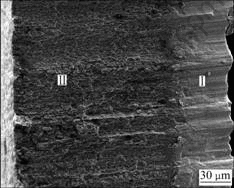
Fig.3 Cross-section micrograph of as-deposited sample
Again, it can be seen that the columnar grains in zone ? grow up at a certain angle to the horizontal plane. This is because the deposition direction of the vapor particles is not vertical to the substrate surface and there forms a small angle between the evaporation path and the normal direction due to the relative position between the crucible and the substrate. The value of the angle is affected by the source-substrate distance and the rotary speed of the substrate.
3.2 Composition and phase
Cross sectional samples from the deposits were investigated using electron microprobe analysis(EPMA) as shown in Fig.4. It is obvious that there exists a given composition segregation in deposited materials and there is a considerable variation in the composition of the materials from the substrate edge to the outer edge.
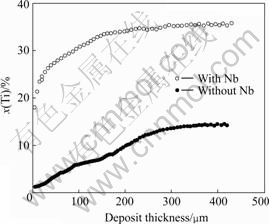
Fig.4 Changes of titanium content along cross-section of deposited materials
This result may be induced by the different saturation vapor pressures of alloy components as explained above, and besides, the variant density of components may have an impact on composition segregation. During the evaporation process, the source materials will reduce gradually and the ambient molten materials will supply evaporation. The supplying rate of Al with a lower density will run faster than that of Ti and lead to the segregation in molten pool, so the evaporation capacity of Ti is less than that of Al at outset, and with evaporation proceeding, the output of Ti will increase gradually. This phenomenon can be eliminated, if a Nb tablet is placed on the ingot surface before evaporation, based on achieving 25%-30% Nb (mass fraction) in the future liquid pool. Then a liquid pool with a higher evaporation temperature is formed at melting of the ingot end face, ensuring transition of the additives into the vapor phase and the condensate without disturbing the pool stability (local boiling or spatter). Nb does not evaporate, and its content in the liquid pool remains practically unchanged[16].
It is obvious that the evaporation process with Nb addition makes it earlier to reach the steady-state compared with that without Nb addition as shown in Fig.4, and the composition of deposit with Nb addition at steady-state is much closer to that of source materials.
The XRD analysis results of annealed samples
obtained from the prepared materials with and without Nb addition in the molten pool during the evaporation process are shown in Fig.5. XRD pattern shows that the constitutive phase of sample without Nb addition during the preparation is TiAl3, which means that the content of Al of deposit is above that of source; while the sample prepared with Nb addition is composed of γ-TiAl and α2-Ti3Al phases, which means that Nb addition during the preparation can increase the evaporation speed of Ti, and make it earlier to reach the steady-state without disturbing the pool stability. In addition, no niobium or niobic compounds are detected in the XRD pattern.

Fig.5 XRD patterns of different samples: (a) Without Nb addition; (b) With Nb addition
3.3 Re-evaporation of Al
The substrate temperature achieved during deposition significantly affects the properties and phases of prepared materials. Related researches show that the transition frequency of surface atoms will tend to increase exponentially as the substrate temperature increases from 750 to 1 000 K which avails the diffusion of atoms on substrate surface and the formation of dense materials[17]. When the substrate temperature reaches 1 000 K which is above the melting point of Al, there will form the re-evaporation processing of Al from the substrate (and this process will happen even if it is below the melting point of Al when the vapor pressure is low enough). Over-fast re-evaporation may restrain the deposition of Al and result in the composition deviation between deposits and vapor. The effect on deposition by re-evaporation of Al can be computed from the published data as discussed below.
The rate of evaporation of a pure element in vacuum is given by Langmuir based on kinetic theory of gases and the concept of dynamic equilibrium [18]. This relationship has also been applied to alloy evaporation with the assumption that each species in the solution will evaporate at a rate consistent with its equilibrium partial pressure over the solution. For a component present in a solution, the surface evaporation rate can be given as follows[19]:
![]() (1)
(1)
where
![]() (2)
(2)
Mi is the molar mass of the component i, αi is the evaporation coefficient, T is the evaporation temperature (K) which equals 1 073 K due to the indication by the thermo-couple, K1 and K2 are material-specific constants, and equal 8.33×106 and 3.815×104 respectively, then the surface evaporation rate can be gained as 1.87×10-14 g/(cm2·s).
The specific evaporating capacity (Q1) and the specific depositing capacity (Q2) can be written as:
Q1=αv1?t?s (3)
Q2=αD1?t?s?ρ (4)
where αD1 is the deposition rate on the assumption that the condensation coefficient is 1, the values of αD1 varies from 1.67×10-9 m/s to 1.67×10-5 m/s according to the external condition, ρ is the density of Al which equals 2.7×103 kg/m3, t is the unit time and s is the unit area. Eqns.(3) and (4) are combined to obtain the variation range of Q2/Q1 is 2.41×108-2.41×1012. The results show that the re-evaporation capacity is far below the deposition capacity, hence the effect on deposit by re-evaporation of Al could be neglected.
4 Conclusions
1) TiAl sheet with dimensions of 450 mm×450 mm×0.2 mm has been prepared by electron beam physical vapor deposition (EB-PVD) technology.
2) Nb addition can potentially have an advantageous effect on alloy evaporation pools, and the material prepared with Nb addition into pool is composed of γ and α2 phases which are closer to the phases composition of the source.
3) Small grain sizes about 200-500 nm were gained due to the high cooling velocity of EB-PVD technology, and there existed equiaxed crystal and columnar crystal along the cross-section resulting from the transformation latent heats released during the transition course of atoms from gaseous state to solid state.
4) Calculation result of the ratio of re-evaporating capacity with depositing capacity of Al on the substrate shows that effect on deposit by re-evaporation of Al could be neglected.
References
[1] AI Tao-tao, WANG Fen, ZHU Jian-feng. Microstructure and mechanical properties of Al2O3/TiAl composite [J]. Trans Nonferrous Met Soc China, 2006, 16(s2): s1924-s1927.
[2] QU H P, WANG H M. Microstructure and mechanical properties of laser melting deposited γ-TiAl intermetallic alloys [J]. Mater Sci Eng A, 2007, 466(1/2): 187-194.
[3] RAHAMAN M N, DUTTON R E, SEMIATIN S L. Fabrication of dense thin sheets of γ-TiAl by hot isostatic pressing of tape-cast monotapes [J]. Mater Sci Eng A, 2003, 360(1/2): 169-175.
[4] LUO Jian-guo. Processing gamma-based TiAl sheet materials by cyclic cold roll bonding and annealing of elemental titanium and aluminum foils [J]. Mater Sci Eng A, 2006, 433(1/2): 334-342.
[5] DRAPER S L, KRAUSE D. Development and evaluation of TiAl sheet structures for hypersonic applications [J]. Mater Sci Eng A, 2007, 464(1/2): 330-342.
[6] LAPIN J. Creep behaviour of a cast TiAl-based alloy for industrial applications [J]. Intermetallics, 2006, 14(2): 115-122.
[7] ADAMS A G, RAHAMAN M N, DUTTON R E. Microstructure of dense thin sheets of γ-TiAl fabricated by hot isostatic pressing of tape-cast monotapes [J]. Mater Sci Eng, 2007, In Press.
[8] SENKOV O N, UCHIC M D. Microstructure evolution during annealing of an amorphous TiAl sheet [J]. Mater Sci Eng A, 2003, 340(1/2): 216-224.
[9] GUAN Chun-long, HE Xiao-dong. Study on microstructure and properties of a Ni-Cr-Al alloy by EB-PVD [J]. Journal of Aeronautical Materials, 2005, 25(1): 11-14.
[10] MA Li, SUN Yue, HE Xiao-dong. Research on ultra-thin high temperature structure materials achieved by EB-PVD [J]. Materials Review, 2006, 20(11): 100-103.
[11] MA Pei-yan, FU Zheng-yi. The latest research on the microlaminated structural materials [J]. Mater Sci Eng, 2002, 20(4): 589-593.
[12] GUAN Chun-long, HE Xiao-dong. Preparation and mechanical properties of Ni-Cr-Al alloy by EB-PVD [J]. Trans Nonferrous Met Soc China, 2005, 15(2): 275-279.
[13] XU Hui-bin, GONG Sheng-kai. Preparation of thermal barrier coatings for gas turbine blades by EB-PVD [J]. Thin Solid Films, 1998, 334(1/2): 98-102.
[14] TANG Wei-zhong. Manufacture principle, technology and application of thin film materials [M]. Beijing: Metallurgical Industry Press, 1998: 1-323.
[15] MOVCHEN B A, DEMCHISHIN A V. Study of structure and properties of thick vacuum condensates of nickel, titanium, tungsten, aluminum oxide and zirconium dioxide [J]. Fiz Metal Metalloved, 1969, 28: 83-90.
[16] MA Li, HE Xiao-dong, HU Zhao-hui. Optimum design, microstructure and mechanical properties of Ti/Ti3Al multi-layered materials [J]. Materials Science Forum, 2007, 546/549: 1575-1580.
[17] SHAN Ying-chun, HE Xiao-dong. Effect of substrate temperature on material establishment in EB-PVD technology [J]. Aerospace Materials and Technology, 2006, 36(1): 41-44.
[18] TEUVO S, ADAMS C M. Kinetics and thermodynamics in continuous electron-beam evaporation of binary alloys [J]. J Vac Sci Technol, 1970, 7(6): s22-s29.
[19] GUO Hong-bo. Hot-fatigue behaviors and failure mechanisms of gradient thermal barrier coating by EB-PVD [D]. Beijing: Beijing University of Aeronautics and Astronautics, 2003.
Foundation item: Project(NCET2004) supported by the Program for New Century Excellent Talents in University, China
Corresponding author: MA Li; Tel: +86-451-86419219; E-mail: mali2008@hit.edu.cn
(Edited by PENG Chao-qun)


